Welcome to the Recombinant Antibody Network
Latest Publications
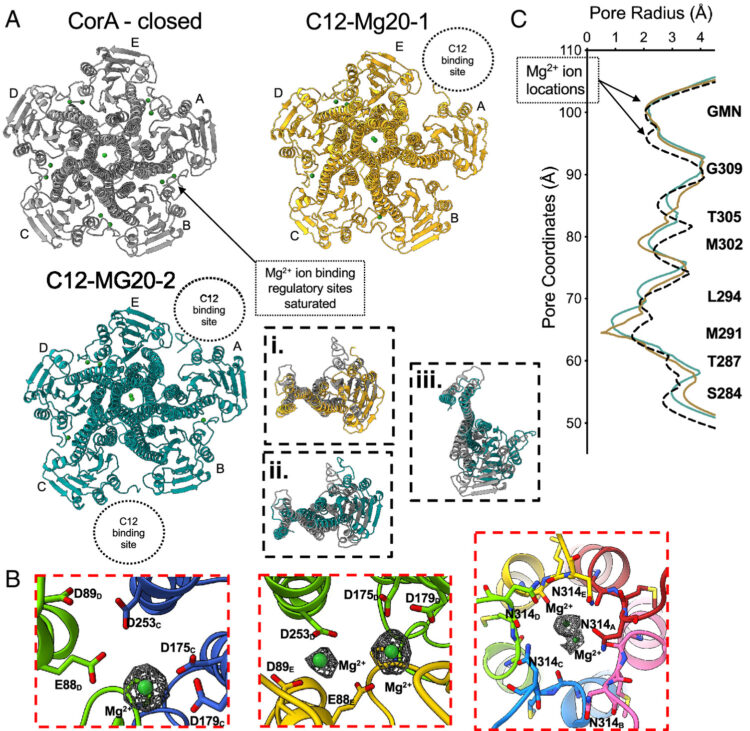
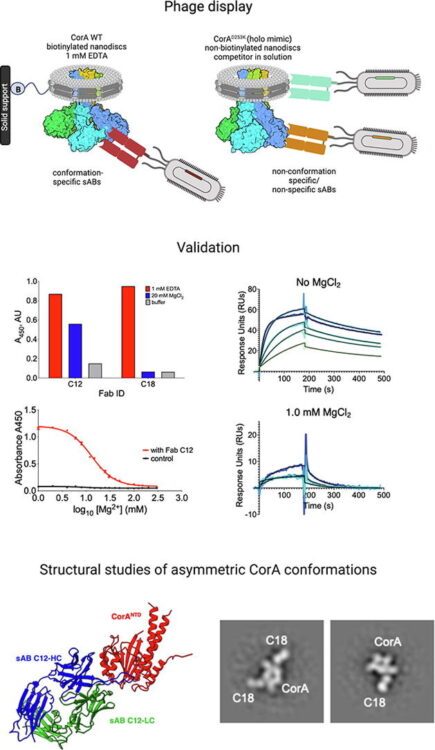
Conformational ensembles of the magnesium channel CorA reveal structural basis for channel gating Journal Article
In: Proc Natl Acad Sci U S A, vol. 123, no. 8, pp. e2512532123, 2026, ISSN: 1091-6490.
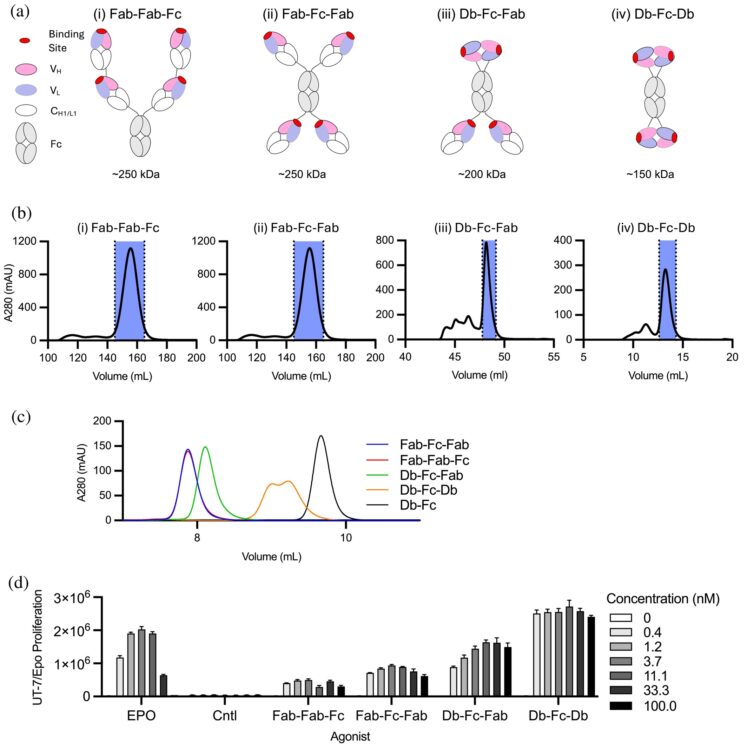
Tetravalent antibodies are more potent and efficacious erythropoiesis-stimulating agents than erythropoietin in vivo Journal Article
In: Protein Sci, vol. 35, no. 2, pp. e70462, 2026, ISSN: 1469-896X.
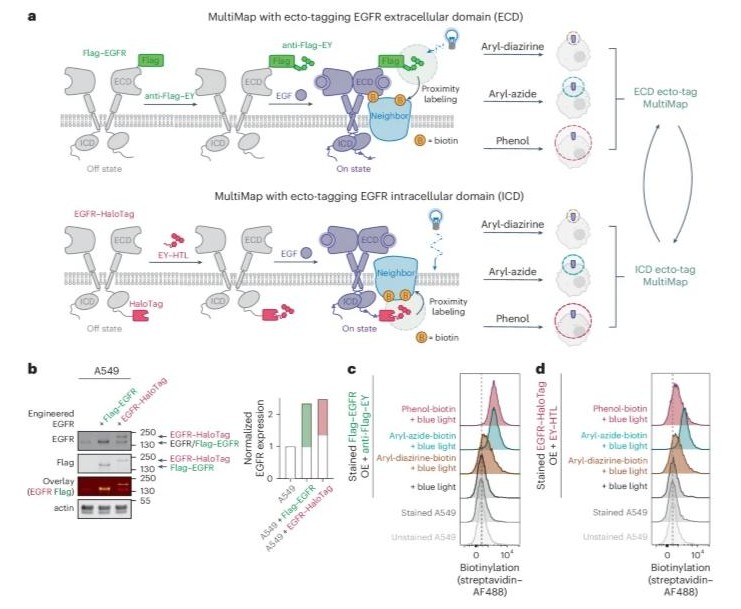
Temporal photoproximity labeling of ligand-activated EGFR neighborhoods using MultiMap Journal Article
In: Nat Chem Biol, vol. 22, no. 2, pp. 192–204, 2026, ISSN: 1552-4469.
Latest News
Recombinant Antibody Network Partners with Bristol Myers Squibb to Develop Novel Therapies
The Recombinant Antibody Network (RAN), a consortium comprising research groups from UC San Francisco, the…
Absolute Antibody Partners with the Recombinant Antibody Network to Facilitate Access to Engineered Recombinant Antibodies
Absolute Antibody Ltd., an industry-leading provider of recombinant antibody products and services, has announced a…
RAN to collaborate with Celgene on cancer therapeutics development
The RAN has recently signed a 3-year $25M agreement with the Celgene Corporation to develop next-generation,…
