Publications: 2019
2019
Dutka, Przemyslaw; Mukherjee, Somnath; Gao, Xiang; Kang, Yanyong; de Waal, Parker W; Wang, Lei; Zhuang, Youwen; Melcher, Karsten; Zhang, Cheng; Xu, H Eric; Kossiakoff, Anthony A
Development of “Plug and Play” Fiducial Marks for Structural Studies of GPCR Signaling Complexes by Single-Particle Cryo-EM Journal Article
In: Structure, vol. 27, no. 12, pp. 1862–1874.e7, 2019, ISSN: 1878-4186.
@article{pmid31669042,
title = {Development of “Plug and Play” Fiducial Marks for Structural Studies of GPCR Signaling Complexes by Single-Particle Cryo-EM},
author = {Przemyslaw Dutka and Somnath Mukherjee and Xiang Gao and Yanyong Kang and Parker W de Waal and Lei Wang and Youwen Zhuang and Karsten Melcher and Cheng Zhang and H Eric Xu and Anthony A Kossiakoff},
doi = {10.1016/j.str.2019.10.004},
issn = {1878-4186},
year = {2019},
date = {2019-12-01},
urldate = {2019-12-01},
journal = {Structure},
volume = {27},
number = {12},
pages = {1862--1874.e7},
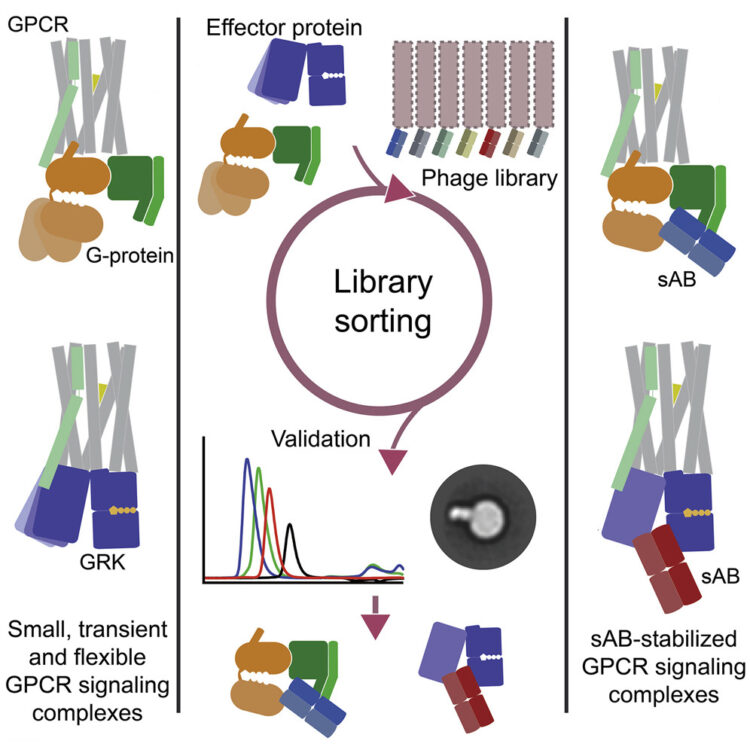
abstract = {"Universal" synthetic antibody (sAB)-based fiducial marks have been generated by customized phage display selections to facilitate the rapid structure determination of G protein-coupled receptor (GPCR) signaling complexes by single-particle cryo-electron microscopy (SP cryo-EM). sABs were generated to the two major G protein subclasses: trimeric G and G, as well as mini-G, and were tested to ensure binding in the context of their cognate GPCRs. Epitope binning revealed that multiple distinct epitopes exist for each G(αβγ) protein. Several Gβγ-specific sABs, cross-reactive between trimeric G and G, were identified suggesting they could be used across all subclasses in a "plug and play" fashion. sABs were also generated to a representative of another class of GPCR signaling partner, G protein receptor kinase 1 (GRK1) and evaluated further, supporting the generalizability of the approach. EM data suggested that the subclass-specific sABs provide effective single and dual fiducials for multiple GPCR signaling complexes.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Kim, Jonathan; Tan, Yong Zi; Wicht, Kathryn J; Erramilli, Satchal K; Dhingra, Satish K; Okombo, John; Vendome, Jeremie; Hagenah, Laura M; Giacometti, Sabrina I; Warren, Audrey L; Nosol, Kamil; Roepe, Paul D; Potter, Clinton S; Carragher, Bridget; Kossiakoff, Anthony A; Quick, Matthias; Fidock, David A; Mancia, Filippo
Structure and drug resistance of the Plasmodium falciparum transporter PfCRT Journal Article
In: Nature, vol. 576, no. 7786, pp. 315–320, 2019, ISSN: 1476-4687.
@article{pmid31776516,
title = {Structure and drug resistance of the Plasmodium falciparum transporter PfCRT},
author = {Jonathan Kim and Yong Zi Tan and Kathryn J Wicht and Satchal K Erramilli and Satish K Dhingra and John Okombo and Jeremie Vendome and Laura M Hagenah and Sabrina I Giacometti and Audrey L Warren and Kamil Nosol and Paul D Roepe and Clinton S Potter and Bridget Carragher and Anthony A Kossiakoff and Matthias Quick and David A Fidock and Filippo Mancia},
doi = {10.1038/s41586-019-1795-x},
issn = {1476-4687},
year = {2019},
date = {2019-12-01},
urldate = {2019-12-01},
journal = {Nature},
volume = {576},
number = {7786},
pages = {315--320},
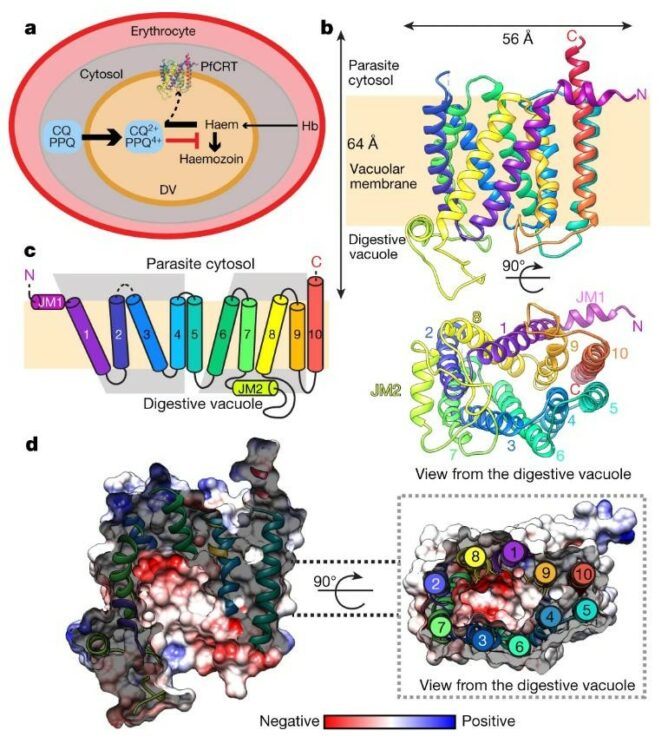
abstract = {The emergence and spread of drug-resistant Plasmodium falciparum impedes global efforts to control and eliminate malaria. For decades, treatment of malaria has relied on chloroquine (CQ), a safe and affordable 4-aminoquinoline that was highly effective against intra-erythrocytic asexual blood-stage parasites, until resistance arose in Southeast Asia and South America and spread worldwide. Clinical resistance to the chemically related current first-line combination drug piperaquine (PPQ) has now emerged regionally, reducing its efficacy. Resistance to CQ and PPQ has been associated with distinct sets of point mutations in the P. falciparum CQ-resistance transporter PfCRT, a 49-kDa member of the drug/metabolite transporter superfamily that traverses the membrane of the acidic digestive vacuole of the parasite. Here we present the structure, at 3.2 Å resolution, of the PfCRT isoform of CQ-resistant, PPQ-sensitive South American 7G8 parasites, using single-particle cryo-electron microscopy and antigen-binding fragment technology. Mutations that contribute to CQ and PPQ resistance localize primarily to moderately conserved sites on distinct helices that line a central negatively charged cavity, indicating that this cavity is the principal site of interaction with the positively charged CQ and PPQ. Binding and transport studies reveal that the 7G8 isoform binds both drugs with comparable affinities, and that these drugs are mutually competitive. The 7G8 isoform transports CQ in a membrane potential- and pH-dependent manner, consistent with an active efflux mechanism that drives CQ resistance, but does not transport PPQ. Functional studies on the newly emerging PfCRT F145I and C350R mutations, associated with decreased PPQ susceptibility in Asia and South America, respectively, reveal their ability to mediate PPQ transport in 7G8 variant proteins and to confer resistance in gene-edited parasites. Structural, functional and in silico analyses suggest that distinct mechanistic features mediate the resistance to CQ and PPQ in PfCRT variants. These data provide atomic-level insights into the molecular mechanism of this key mediator of antimalarial treatment failures.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
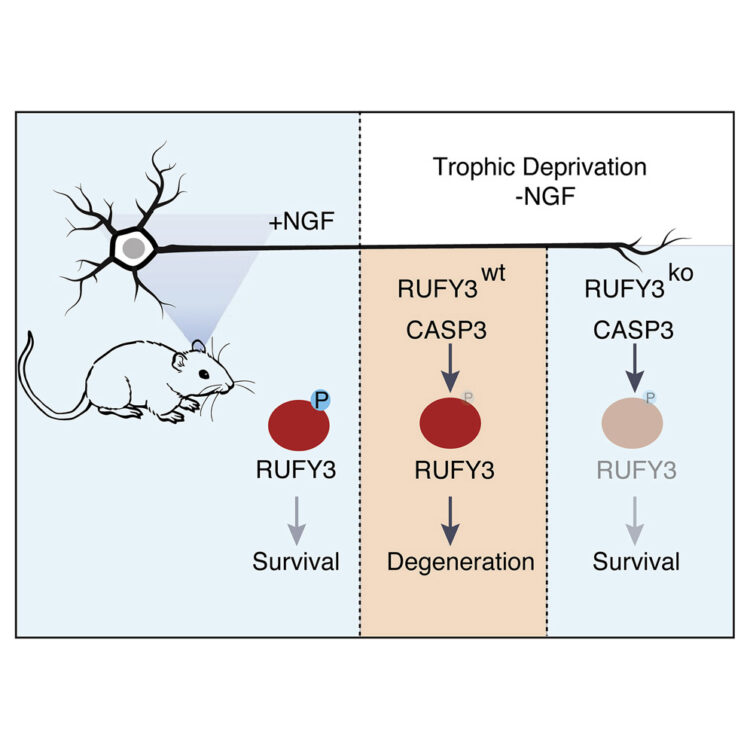
Hertz, Nicholas T.; Adams, Eliza L.; Weber, Ross A.; Shen, Rebecca J.; O’Rourke, Melanie K.; Simon, David J.; Zebroski, Henry; Olsen, Olav; Morgan, Charles W.; Mileur, Trevor R.; Hitchcock, Angela M.; Armstrong, Nicholas A. Sinnott; Wainberg, Michael; Bassik, Michael C.; Molina, Henrik; Wells, James A.; Tessier-Lavigne, Marc
Neuronally Enriched RUFY3 Is Required for Caspase-Mediated Axon Degeneration Journal Article
In: Neuron, vol. 103, no. 3, pp. 412–422.e4, 2019, ISSN: 0896-6273.
@article{Hertz2019,
title = {Neuronally Enriched RUFY3 Is Required for Caspase-Mediated Axon Degeneration},
author = {Nicholas T. Hertz and Eliza L. Adams and Ross A. Weber and Rebecca J. Shen and Melanie K. O’Rourke and David J. Simon and Henry Zebroski and Olav Olsen and Charles W. Morgan and Trevor R. Mileur and Angela M. Hitchcock and Nicholas A. Sinnott Armstrong and Michael Wainberg and Michael C. Bassik and Henrik Molina and James A. Wells and Marc Tessier-Lavigne},
doi = {10.1016/j.neuron.2019.05.030},
issn = {0896-6273},
year = {2019},
date = {2019-08-00},
urldate = {2019-08-00},
journal = {Neuron},
volume = {103},
number = {3},
pages = {412--422.e4},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Yueh, Christine; Rettenmaier, Justin; Xia, Bing; Hall, David R.; Alekseenko, Andrey; Porter, Kathryn A.; Barkovich, Krister; Keseru, Gyorgy; Whitty, Adrian; Wells, James A.; Vajda, Sandor; Kozakov, Dima
Kinase Atlas: Druggability Analysis of Potential Allosteric Sites in Kinases Journal Article
In: J. Med. Chem., vol. 62, no. 14, pp. 6512–6524, 2019, ISSN: 1520-4804.
@article{Yueh2019,
title = {Kinase Atlas: Druggability Analysis of Potential Allosteric Sites in Kinases},
author = {Christine Yueh and Justin Rettenmaier and Bing Xia and David R. Hall and Andrey Alekseenko and Kathryn A. Porter and Krister Barkovich and Gyorgy Keseru and Adrian Whitty and James A. Wells and Sandor Vajda and Dima Kozakov},
doi = {10.1021/acs.jmedchem.9b00089},
issn = {1520-4804},
year = {2019},
date = {2019-07-25},
urldate = {2019-07-25},
journal = {J. Med. Chem.},
volume = {62},
number = {14},
pages = {6512--6524},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Farcasanu, Mara; Wang, Andrew G; Uchański, Tomasz; Bailey, Lucas J; Yue, Jiping; Chen, Zhaochun; Wu, Xiaoyang; Kossiakoff, Anthony; Tang, Wei-Jen
Rapid Discovery and Characterization of Synthetic Neutralizing Antibodies against Anthrax Edema Toxin Journal Article
In: Biochemistry, vol. 58, no. 27, pp. 2996–3004, 2019, ISSN: 1520-4995.
@article{pmid31243996,
title = {Rapid Discovery and Characterization of Synthetic Neutralizing Antibodies against Anthrax Edema Toxin},
author = {Mara Farcasanu and Andrew G Wang and Tomasz Uchański and Lucas J Bailey and Jiping Yue and Zhaochun Chen and Xiaoyang Wu and Anthony Kossiakoff and Wei-Jen Tang},
doi = {10.1021/acs.biochem.9b00184},
issn = {1520-4995},
year = {2019},
date = {2019-07-01},
urldate = {2019-07-01},
journal = {Biochemistry},
volume = {58},
number = {27},
pages = {2996--3004},
abstract = {Anthrax, a lethal, weaponizable disease caused by Bacillus anthracis, acts through exotoxins that are primary mediators of systemic toxicity and also targets for neutralization by passive immunotherapy. The ease of engineering B. anthracis strains resistant to established therapy and the historic use of the microbe in bioterrorism present a compelling test case for platforms that permit the rapid and modular development of neutralizing agents. In vitro antigen-binding fragment (Fab) selection offers the advantages of speed, sequence level molecular control, and engineering flexibility compared to traditional monoclonal antibody pipelines. By screening an unbiased, chemically synthetic phage Fab library and characterizing hits in cell-based assays, we identified two high-affinity neutralizing Fabs, A4 and B7, against anthrax edema factor (EF), a key mediator of anthrax pathogenesis. Engineered homodimers of these Fabs exhibited potency comparable to that of the best reported neutralizing monoclonal antibody against EF at preventing EF-induced cyclic AMP production. Using internalization assays in COS cells, B7 was found to block steps prior to EF internalization. This work demonstrates the efficacy of synthetic alternatives to traditional antibody therapeutics against anthrax while also demonstrating a broadly generalizable, rapid, and modular screening pipeline for neutralizing antibody generation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}