Publications: 2012
2012
Crawford, E D; Seaman, J E; Barber, A E; David, D C; Babbitt, P C; Burlingame, A L; Wells, J A
In: Cell Death Differ, vol. 19, no. 12, pp. 2040–2048, 2012, ISSN: 1476-5403.
@article{pmid22918439,
title = {Conservation of caspase substrates across metazoans suggests hierarchical importance of signaling pathways over specific targets and cleavage site motifs in apoptosis},
author = {E D Crawford and J E Seaman and A E Barber and D C David and P C Babbitt and A L Burlingame and J A Wells},
doi = {10.1038/cdd.2012.99},
issn = {1476-5403},
year = {2012},
date = {2012-12-01},
urldate = {2012-12-01},
journal = {Cell Death Differ},
volume = {19},
number = {12},
pages = {2040--2048},
abstract = {Caspases, cysteine proteases with aspartate specificity, are key players in programmed cell death across the metazoan lineage. Hundreds of apoptotic caspase substrates have been identified in human cells. Some have been extensively characterized, revealing key functional nodes for apoptosis signaling and important drug targets in cancer. But the functional significance of most cuts remains mysterious. We set out to better understand the importance of caspase cleavage specificity in apoptosis by asking which cleavage events are conserved across metazoan model species. Using N-terminal labeling followed by mass spectrometry, we identified 257 caspase cleavage sites in mouse, 130 in Drosophila, and 50 in Caenorhabditis elegans. The large majority of the caspase cut sites identified in mouse proteins were found conserved in human orthologs. However, while many of the same proteins targeted in the more distantly related species were cleaved in human orthologs, the exact sites were often different. Furthermore, similar functional pathways are targeted by caspases in all four species. Our data suggest a model for the evolution of apoptotic caspase specificity that highlights the hierarchical importance of functional pathways over specific proteins, and proteins over their specific cleavage site motifs.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
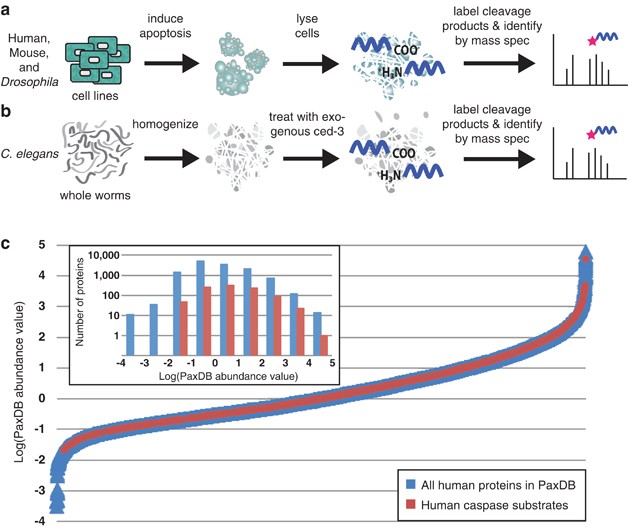
Zorn, Julie A; Wolan, Dennis W; Agard, Nicholas J; Wells, James A
Fibrils colocalize caspase-3 with procaspase-3 to foster maturation Journal Article
In: J Biol Chem, vol. 287, no. 40, pp. 33781–33795, 2012, ISSN: 1083-351X.
@article{pmid22872644,
title = {Fibrils colocalize caspase-3 with procaspase-3 to foster maturation},
author = {Julie A Zorn and Dennis W Wolan and Nicholas J Agard and James A Wells},
doi = {10.1074/jbc.M112.386128},
issn = {1083-351X},
year = {2012},
date = {2012-09-01},
urldate = {2012-09-01},
journal = {J Biol Chem},
volume = {287},
number = {40},
pages = {33781--33795},
abstract = {Most proteases are expressed as inactive precursors, or zymogens, that become activated by limited proteolysis. We previously identified a small molecule, termed 1541, that dramatically promotes the maturation of the zymogen, procaspase-3, to its mature form, caspase-3. Surprisingly, compound 1541 self-assembles into nanofibrils, and localization of procaspase-3 to the fibrils promotes activation. Here, we interrogate the biochemical mechanism of procaspase-3 activation on 1541 fibrils in addition to proteogenic amyloid-β(1-40) fibrils. In contrast to previous reports, we find no evidence that procaspase-3 alone is capable of self-activation, consistent with its fate-determining role in executing apoptosis. In fact, mature caspase-3 is >10(7)-fold more active than procaspase-3, making this proenzyme a remarkably inactive zymogen. However, we also show that fibril-induced colocalization of trace amounts of caspase-3 or other initiator proteases with procaspase-3 dramatically stimulates maturation of the proenzyme in vitro. Thus, similar to known cellular signaling complexes, these synthetic or natural fibrils can serve as platforms to concentrate procaspase-3 for trans-activation by upstream proteases.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
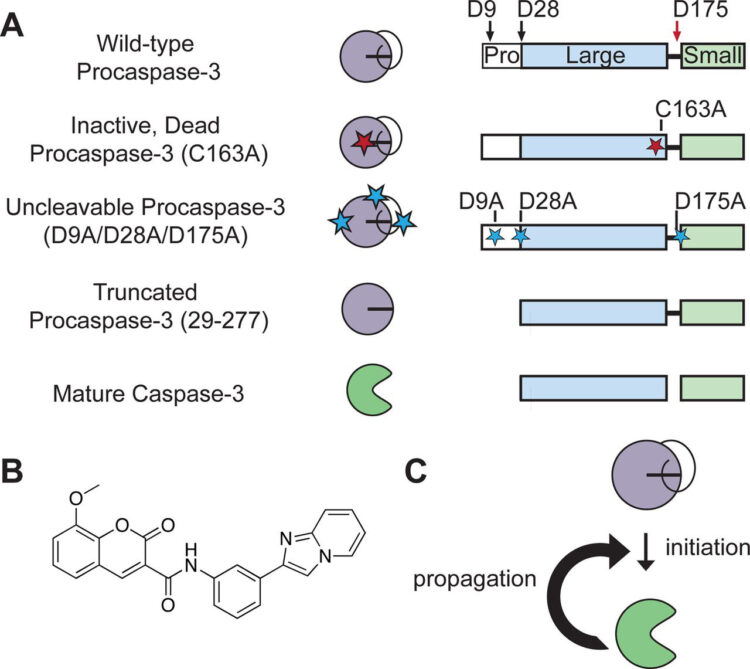
Shimbo, Kazutaka; Hsu, Gerald W; Nguyen, Huy; Mahrus, Sami; Trinidad, Jonathan C; Burlingame, Alma L; Wells, James A
Quantitative profiling of caspase-cleaved substrates reveals different drug-induced and cell-type patterns in apoptosis Journal Article
In: Proc Natl Acad Sci U S A, vol. 109, no. 31, pp. 12432–12437, 2012, ISSN: 1091-6490.
@article{pmid22802652,
title = {Quantitative profiling of caspase-cleaved substrates reveals different drug-induced and cell-type patterns in apoptosis},
author = {Kazutaka Shimbo and Gerald W Hsu and Huy Nguyen and Sami Mahrus and Jonathan C Trinidad and Alma L Burlingame and James A Wells},
doi = {10.1073/pnas.1208616109},
issn = {1091-6490},
year = {2012},
date = {2012-07-01},
urldate = {2012-07-01},
journal = {Proc Natl Acad Sci U S A},
volume = {109},
number = {31},
pages = {12432--12437},
abstract = {Proapoptotic drugs are a mainstay of cancer drug treatment. These drugs stress cells and ultimately trigger the activation of caspases, cysteine-class proteases that cleave after aspartic acid and deconstruct the cell. It is well known that cells respond differently to proapoptotic cancer drug treatments. Here, using a global and unbiased quantitative N-terminomics technology, we show that ~500 products of caspase cleavage and their kinetics vary dramatically between cell type and cytotoxic drug treatment. It is likely that variations arise from differences in baseline proteome composition of the cell type and the alterations induced by drug treatments to yield a unique cohort of proteins that caspases finally target. Many targets are specific to both drug treatment and cell type, providing candidate-specific biomarkers for apoptosis. For example, in multiple myeloma cells treated with the proteasome inhibitor bortezomib, levels of activating transcription factor-4 increase dramatically early in drug treatment and then decrease upon cleavage by activated caspases. Thus, caspase-derived cleavage products are a sensitive reflection of cell-type and drug-induced stress, and provide useful fingerprints for mechanisms of drug action and response.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
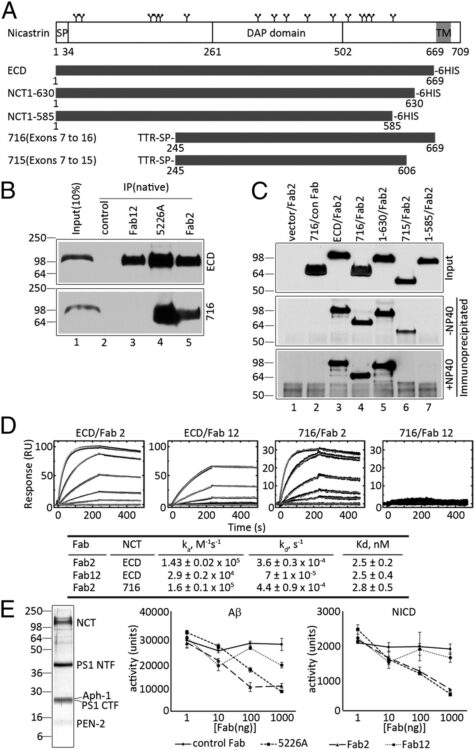
Zhang, Xulun; Hoey, Robert J; Lin, Guoqing; Koide, Akiko; Leung, Brenda; Ahn, Kwangwook; Dolios, Georgia; Paduch, Marcin; Ikeuchi, Takeshi; Wang, Rong; Li, Yue-Ming; Koide, Shohei; Sisodia, Sangram S
Identification of a tetratricopeptide repeat-like domain in the nicastrin subunit of γ-secretase using synthetic antibodies Journal Article
In: Proc Natl Acad Sci U S A, vol. 109, no. 22, pp. 8534–8539, 2012, ISSN: 1091-6490.
@article{pmid22586122,
title = {Identification of a tetratricopeptide repeat-like domain in the nicastrin subunit of γ-secretase using synthetic antibodies},
author = {Xulun Zhang and Robert J Hoey and Guoqing Lin and Akiko Koide and Brenda Leung and Kwangwook Ahn and Georgia Dolios and Marcin Paduch and Takeshi Ikeuchi and Rong Wang and Yue-Ming Li and Shohei Koide and Sangram S Sisodia},
doi = {10.1073/pnas.1202691109},
issn = {1091-6490},
year = {2012},
date = {2012-05-01},
urldate = {2012-05-01},
journal = {Proc Natl Acad Sci U S A},
volume = {109},
number = {22},
pages = {8534--8539},
abstract = {The γ-secretase complex, composed of presenilin, anterior-pharynx-defective 1, nicastrin, and presenilin enhancer 2, catalyzes the intramembranous processing of a wide variety of type I membrane proteins, including amyloid precursor protein (APP) and Notch. Earlier studies have revealed that nicastrin, a type I membrane-anchored glycoprotein, plays a role in γ-secretase assembly and trafficking and has been proposed to bind substrates. To gain more insights regarding nicastrin structure and function, we generated a conformation-specific synthetic antibody and used it as a molecular probe to map functional domains within nicastrin ectodomain. The antibody bound to a conformational epitope within a nicastrin segment encompassing residues 245-630 and inhibited the processing of APP and Notch substrates in in vitro γ-secretase activity assays, suggesting that a functional domain pertinent to γ-secretase activity resides within this region. Epitope mapping and database searches revealed the presence of a structured segment, located downstream of the previously identified DAP domain (DYIGS and peptidase; residues 261-502), that is homologous to a tetratricopeptide repeat (TPR) domain commonly involved in peptide recognition. Mutagenesis analyses within the predicted TPR-like domain showed that disruption of the signature helical structure resulted in the loss of γ-secretase activity but not the assembly of the γ-secretase and that Leu571 within the TPR-like domain plays an important role in mediating substrate binding. Taken together, these studies offer provocative insights pertaining to the structural basis for nicastrin function as a "substrate receptor" within the γ-secretase complex.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
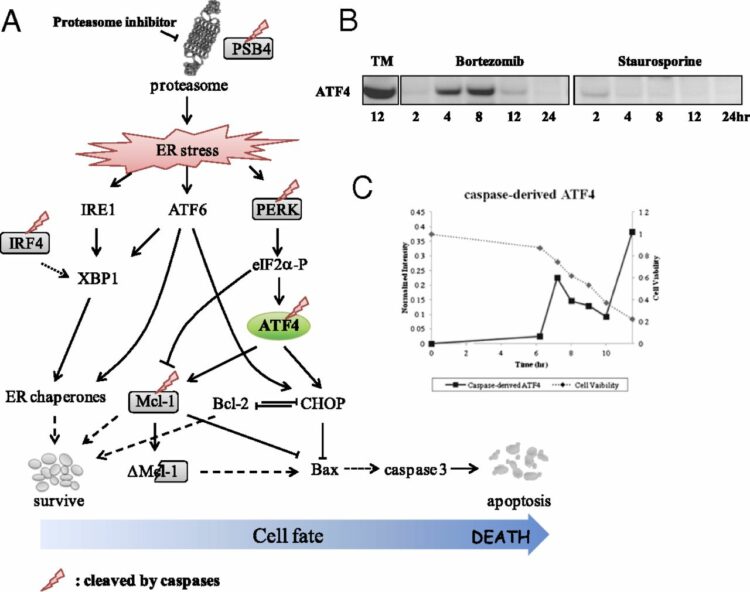
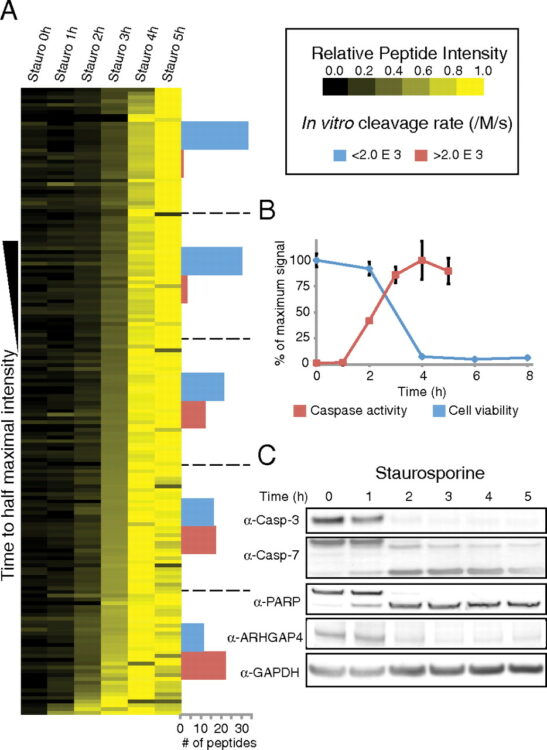
Agard, Nicholas J; Mahrus, Sami; Trinidad, Jonathan C; Lynn, Aenoch; Burlingame, Alma L; Wells, James A
Global kinetic analysis of proteolysis via quantitative targeted proteomics Journal Article
In: Proc Natl Acad Sci U S A, vol. 109, no. 6, pp. 1913–1918, 2012, ISSN: 1091-6490.
@article{pmid22308409,
title = {Global kinetic analysis of proteolysis via quantitative targeted proteomics},
author = {Nicholas J Agard and Sami Mahrus and Jonathan C Trinidad and Aenoch Lynn and Alma L Burlingame and James A Wells},
doi = {10.1073/pnas.1117158109},
issn = {1091-6490},
year = {2012},
date = {2012-02-01},
urldate = {2012-02-01},
journal = {Proc Natl Acad Sci U S A},
volume = {109},
number = {6},
pages = {1913--1918},
abstract = {Mass spectrometry-based proteomics is a powerful tool for identifying hundreds to thousands of posttranslational modifications in complex mixtures. However, it remains enormously challenging to simultaneously assess the intrinsic catalytic efficiencies (k(cat)/K(M)) of these modifications in the context of their natural interactors. Such fundamental enzymological constants are key to determining substrate specificity and for establishing the timing and importance of cellular signaling. Here, we report the use of selected reaction monitoring (SRM) for tracking proteolysis induced by human apoptotic caspases-3, -7, -8, and -9 in lysates and living cells. By following the appearance of the cleaved peptides in lysate as a function of time, we were able to determine hundreds of catalytic efficiencies in parallel. Remarkably, we find the rates of substrate hydrolysis for individual caspases vary greater than 500-fold indicating a sequential process. Moreover, the rank-order of substrate cutting is similar in apoptotic cells, suggesting that cellular structures do not dramatically alter substrate accessibility. Comparisons of extrinsic (TRAIL) and intrinsic (staurosporine) inducers of apoptosis revealed similar substrate profiles, suggesting the final proteolytic demolitions proceed by similarly ordered plans. Certain biological processes were rapidly targeted by the caspases, including multiple components of the endocyotic pathway and miRNA processing machinery. We believe this massively parallel and quantitative label-free approach to obtaining basic enzymological constants will facilitate the study of proteolysis and other posttranslational modifications in complex mixtures.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
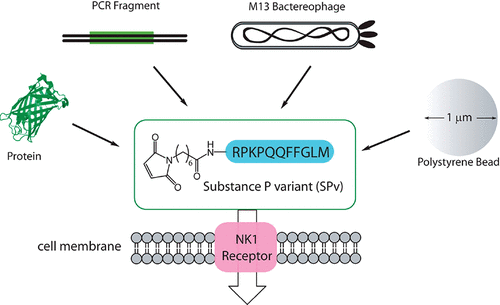
Rizk, Shahir S; Misiura, Agnieszka; Paduch, Marcin; Kossiakoff, Anthony A
Substance P derivatives as versatile tools for specific delivery of various types of biomolecular cargo Journal Article
In: Bioconjug Chem, vol. 23, no. 1, pp. 42–46, 2012, ISSN: 1520-4812.
@article{pmid22175275,
title = {Substance P derivatives as versatile tools for specific delivery of various types of biomolecular cargo},
author = {Shahir S Rizk and Agnieszka Misiura and Marcin Paduch and Anthony A Kossiakoff},
doi = {10.1021/bc200496e},
issn = {1520-4812},
year = {2012},
date = {2012-01-01},
urldate = {2012-01-01},
journal = {Bioconjug Chem},
volume = {23},
number = {1},
pages = {42--46},
abstract = {The use of proteins or nucleic acids as therapeutic agents has been severely hampered by their intrinsic inability to cross the cell membrane. Moreover, common techniques for driving the delivery of macromolecules lack the ability to distinguish between healthy and diseased tissue, precluding their clinical use. Recently, receptor-mediated delivery (RMD) has emerged as a technology with the potential to circumvent the obstacles associated with the delivery of drug targets by utilizing the natural endocytosis of a ligand upon binding to its receptor. Here, we describe the synthesis of variants of substance P (SP), an eleven amino acid neuropeptide ligand of the neurokinin type 1 receptor (NK1R), for the delivery of various types of cargo. The variants of SP were synthesized with an N-terminal maleimide moiety that allows conjugation to surface thiols, resulting in a nonreducible thioether. Cargos lacking an available thiol are conjugated to SP using commercially available cross-linkers. In addition to the delivery of proteins, we expand the use of SP to include nuclear delivery of DNA fragments that are actively expressed in the target cells. We also show that SP can be used to deliver whole bacteriophage particles as well as polystyrene beads up to 1 μm in diameter. The results show the ability of SP to deliver cargo of various sizes and chemical properties that retain their function within the cell. Furthermore, the overexpression of the NK1R in many tumors provides the potential for developing targeted delivery reagents that are specific toward diseased tissue.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}