Publications: 2014
2014
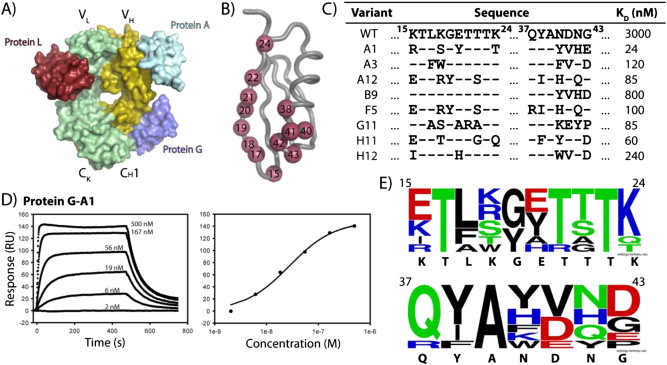
Bailey, Lucas J; Sheehy, Kimberly M; Hoey, Robert J; Schaefer, Zachary P; Ura, Marcin; Kossiakoff, Anthony A
Applications for an engineered Protein-G variant with a pH controllable affinity to antibody fragments Journal Article
In: J Immunol Methods, vol. 415, pp. 24–30, 2014, ISSN: 1872-7905.
@article{pmid25450256,
title = {Applications for an engineered Protein-G variant with a pH controllable affinity to antibody fragments},
author = {Lucas J Bailey and Kimberly M Sheehy and Robert J Hoey and Zachary P Schaefer and Marcin Ura and Anthony A Kossiakoff},
doi = {10.1016/j.jim.2014.10.003},
issn = {1872-7905},
year = {2014},
date = {2014-12-01},
urldate = {2014-12-01},
journal = {J Immunol Methods},
volume = {415},
pages = {24--30},
abstract = {Immunoglobulin binding proteins (IBPs) are broadly used as reagents for the purification and detection of antibodies. Among the IBPs, the most widely used are Protein-A and Protein-G. The C2 domain of Protein-G from Streptococcus is a multi-specific protein domain; it possesses a high affinity (K(D) ~10 nM) for the Fc region of the IgG, but a much lower affinity (KD~low μM) for the constant domain of the antibody fragment (Fab), which limits some of its applications. Here, we describe the engineering of the Protein-G interface using phage display to create Protein-G-A1, a variant with 8 point mutations and an approximately 100-fold improved affinity over the parent domain for the 4D5 Fab scaffold. Protein-G-A1 is capable of robust binding to Fab fragments for numerous applications. Furthermore, we isolated a variant with pH-dependent affinity, demonstrating a 1,000-fold change in affinity from pH7 to 4. Additional rational mutagenesis endowed Protein-G with significantly enhanced stability in basic conditions relative to the parent domain while maintaining high affinity to the Fab. This property is particularly useful to regenerate Protein-G affinity columns. Lastly, the affinity-matured Protein-G-A1 variant was tethered together to produce dimers capable of providing multivalent affinity enhancement to a low affinity antibody fragment-antigen interaction. Engineered Protein-G variants should find widespread application in the use of Fab-based affinity reagents.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
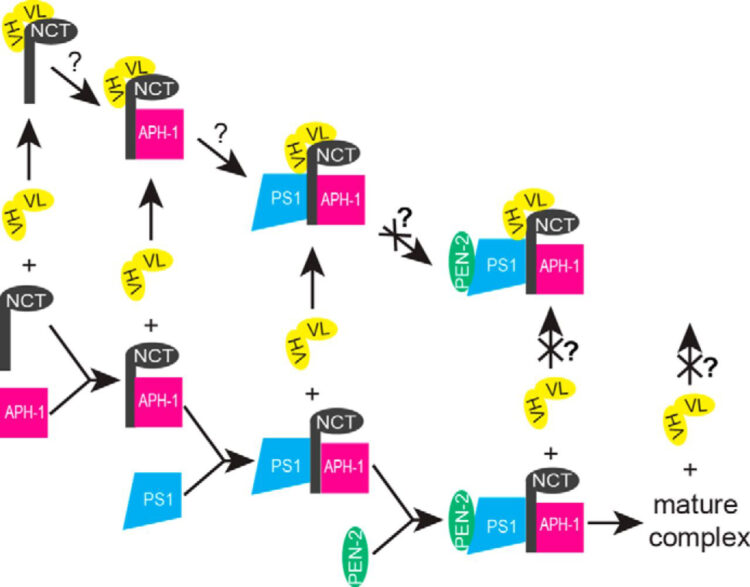
Zhang, Xulun; Hoey, Robert; Koide, Akiko; Dolios, Georgia; Paduch, Marcin; Nguyen, Phuong; Wu, Xianzhong; Li, Yueming; Wagner, Steven L; Wang, Rong; Koide, Shohei; Sisodia, Sangram S
A synthetic antibody fragment targeting nicastrin affects assembly and trafficking of γ-secretase Journal Article
In: J Biol Chem, vol. 289, no. 50, pp. 34851–34861, 2014, ISSN: 1083-351X.
@article{pmid25352592,
title = {A synthetic antibody fragment targeting nicastrin affects assembly and trafficking of γ-secretase},
author = {Xulun Zhang and Robert Hoey and Akiko Koide and Georgia Dolios and Marcin Paduch and Phuong Nguyen and Xianzhong Wu and Yueming Li and Steven L Wagner and Rong Wang and Shohei Koide and Sangram S Sisodia},
doi = {10.1074/jbc.M114.609636},
issn = {1083-351X},
year = {2014},
date = {2014-12-01},
urldate = {2014-12-01},
journal = {J Biol Chem},
volume = {289},
number = {50},
pages = {34851--34861},
abstract = {The γ-secretase complex, composed of presenilin, nicastrin (NCT), anterior pharynx-defective 1 (APH-1), and presenilin enhancer 2 (PEN-2), is assembled in a highly regulated manner and catalyzes the intramembranous proteolysis of many type I membrane proteins, including Notch and amyloid precursor protein. The Notch family of receptors plays important roles in cell fate specification during development and in adult tissues, and aberrant hyperactive Notch signaling causes some forms of cancer. γ-Secretase-mediated processing of Notch at the cell surface results in the generation of the Notch intracellular domain, which associates with several transcriptional coactivators involved in nuclear signaling events. On the other hand, γ-secretase-mediated processing of amyloid precursor protein leads to the production of amyloid β (Aβ) peptides that play an important role in the pathogenesis of Alzheimer disease. We used a phage display approach to identify synthetic antibodies that specifically target NCT and expressed them in the single-chain variable fragment (scFv) format in mammalian cells. We show that expression of a NCT-specific scFv clone, G9, in HEK293 cells decreased the production of the Notch intracellular domain but not the production of amyloid β peptides that occurs in endosomal and recycling compartments. Biochemical studies revealed that scFvG9 impairs the maturation of NCT by associating with immature forms of NCT and, consequently, prevents its association with the other components of the γ-secretase complex, leading to degradation of these molecules. The reduced cell surface levels of mature γ-secretase complexes, in turn, compromise the intramembranous processing of Notch.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
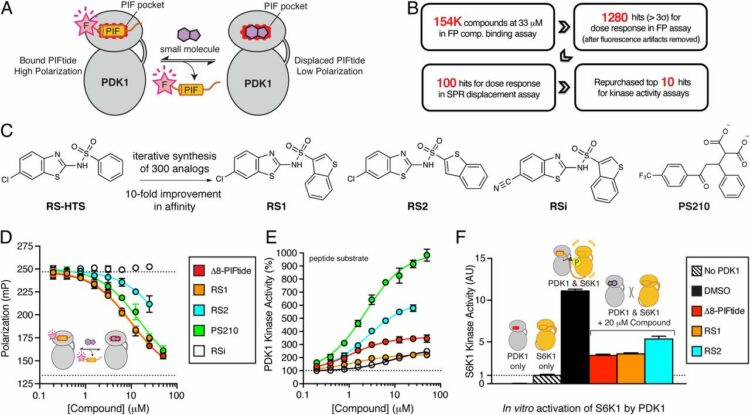
Rettenmaier, T Justin; Sadowsky, Jack D; Thomsen, Nathan D; Chen, Steven C; Doak, Allison K; Arkin, Michelle R; Wells, James A
A small-molecule mimic of a peptide docking motif inhibits the protein kinase PDK1 Journal Article
In: Proc Natl Acad Sci U S A, vol. 111, no. 52, pp. 18590–18595, 2014, ISSN: 1091-6490.
@article{pmid25518860,
title = {A small-molecule mimic of a peptide docking motif inhibits the protein kinase PDK1},
author = {T Justin Rettenmaier and Jack D Sadowsky and Nathan D Thomsen and Steven C Chen and Allison K Doak and Michelle R Arkin and James A Wells},
doi = {10.1073/pnas.1415365112},
issn = {1091-6490},
year = {2014},
date = {2014-12-01},
urldate = {2014-12-01},
journal = {Proc Natl Acad Sci U S A},
volume = {111},
number = {52},
pages = {18590--18595},
abstract = {There is great interest in developing selective protein kinase inhibitors by targeting allosteric sites, but these sites often involve protein-protein or protein-peptide interfaces that are very challenging to target with small molecules. Here we present a systematic approach to targeting a functionally conserved allosteric site on the protein kinase PDK1 called the PDK1-interacting fragment (PIF)tide-binding site, or PIF pocket. More than two dozen prosurvival and progrowth kinases dock a conserved peptide tail into this binding site, which recruits them to PDK1 to become activated. Using a site-directed chemical screen, we identified and chemically optimized ligand-efficient, selective, and cell-penetrant small molecules (molecular weight ∼ 380 Da) that compete with the peptide docking motif for binding to PDK1. We solved the first high-resolution structure of a peptide docking motif (PIFtide) bound to PDK1 and mapped binding energy hot spots using mutational analysis. We then solved structures of PDK1 bound to the allosteric small molecules, which revealed a binding mode that remarkably mimics three of five hot-spot residues in PIFtide. These allosteric small molecules are substrate-selective PDK1 inhibitors when used as single agents, but when combined with an ATP-competitive inhibitor, they completely suppress the activation of the downstream kinases. This work provides a promising new scaffold for the development of high-affinity PIF pocket ligands, which may be used to enhance the anticancer activity of existing PDK1 inhibitors. Moreover, our results provide further impetus for exploring the helix αC patches of other protein kinases as potential therapeutic targets even though they involve protein-protein interfaces.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
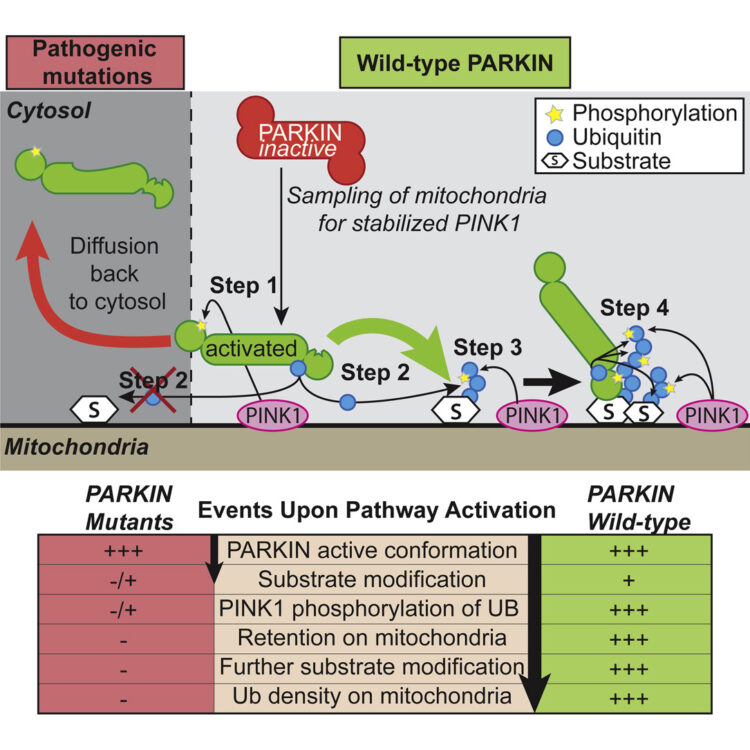
Ordureau, Alban; Sarraf, Shireen A; Duda, David M; Heo, Jin-Mi; Jedrychowski, Mark P; Sviderskiy, Vladislav O; Olszewski, Jennifer L; Koerber, James T; Xie, Tiao; Beausoleil, Sean A; Wells, James A; Gygi, Steven P; Schulman, Brenda A; Harper, J Wade
Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis Journal Article
In: Mol Cell, vol. 56, no. 3, pp. 360–375, 2014, ISSN: 1097-4164.
@article{pmid25284222,
title = {Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis},
author = {Alban Ordureau and Shireen A Sarraf and David M Duda and Jin-Mi Heo and Mark P Jedrychowski and Vladislav O Sviderskiy and Jennifer L Olszewski and James T Koerber and Tiao Xie and Sean A Beausoleil and James A Wells and Steven P Gygi and Brenda A Schulman and J Wade Harper},
doi = {10.1016/j.molcel.2014.09.007},
issn = {1097-4164},
year = {2014},
date = {2014-11-01},
urldate = {2014-11-01},
journal = {Mol Cell},
volume = {56},
number = {3},
pages = {360--375},
abstract = {Phosphorylation is often used to promote protein ubiquitylation, yet we rarely understand quantitatively how ligase activation and ubiquitin (UB) chain assembly are integrated with phosphoregulation. Here we employ quantitative proteomics and live-cell imaging to dissect individual steps in the PINK1 kinase-PARKIN UB ligase mitochondrial control pathway disrupted in Parkinson's disease. PINK1 plays a dual role by phosphorylating PARKIN on its UB-like domain and poly-UB chains on mitochondria. PARKIN activation by PINK1 produces canonical and noncanonical UB chains on mitochondria, and PARKIN-dependent chain assembly is required for accumulation of poly-phospho-UB (poly-p-UB) on mitochondria. In vitro, PINK1 directly activates PARKIN's ability to assemble canonical and noncanonical UB chains and promotes association of PARKIN with both p-UB and poly-p-UB. Our data reveal a feedforward mechanism that explains how PINK1 phosphorylation of both PARKIN and poly-UB chains synthesized by PARKIN drives a program of PARKIN recruitment and mitochondrial ubiquitylation in response to mitochondrial damage.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
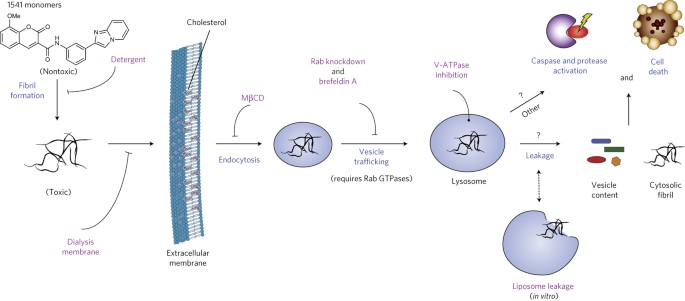
Julien, Olivier; Kampmann, Martin; Bassik, Michael C; Zorn, Julie A; Venditto, Vincent J; Shimbo, Kazutaka; Agard, Nicholas J; Shimada, Kenichi; Rheingold, Arnold L; Stockwell, Brent R; Weissman, Jonathan S; Wells, James A
Unraveling the mechanism of cell death induced by chemical fibrils Journal Article
In: Nat Chem Biol, vol. 10, no. 11, pp. 969–976, 2014, ISSN: 1552-4469.
@article{pmid25262416,
title = {Unraveling the mechanism of cell death induced by chemical fibrils},
author = {Olivier Julien and Martin Kampmann and Michael C Bassik and Julie A Zorn and Vincent J Venditto and Kazutaka Shimbo and Nicholas J Agard and Kenichi Shimada and Arnold L Rheingold and Brent R Stockwell and Jonathan S Weissman and James A Wells},
doi = {10.1038/nchembio.1639},
issn = {1552-4469},
year = {2014},
date = {2014-11-01},
urldate = {2014-11-01},
journal = {Nat Chem Biol},
volume = {10},
number = {11},
pages = {969--976},
abstract = {We previously discovered a small-molecule inducer of cell death, named 1541, that noncovalently self-assembles into chemical fibrils ('chemi-fibrils') and activates procaspase-3 in vitro. We report here that 1541-induced cell death is caused by the fibrillar rather than the soluble form of the drug. A short hairpin RNA screen reveals that knockdown of genes involved in endocytosis, vesicle trafficking and lysosomal acidification causes partial 1541 resistance. We confirm the role of these pathways using pharmacological inhibitors. Microscopy shows that the fluorescent chemi-fibrils accumulate in punctae inside cells that partially colocalize with lysosomes. Notably, the chemi-fibrils bind and induce liposome leakage in vitro, suggesting they may do the same in cells. The chemi-fibrils induce extensive proteolysis including caspase substrates, yet modulatory profiling reveals that chemi-fibrils form a distinct class from existing inducers of cell death. The chemi-fibrils share similarities with proteinaceous fibrils and may provide insight into their mechanism of cellular toxicity.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
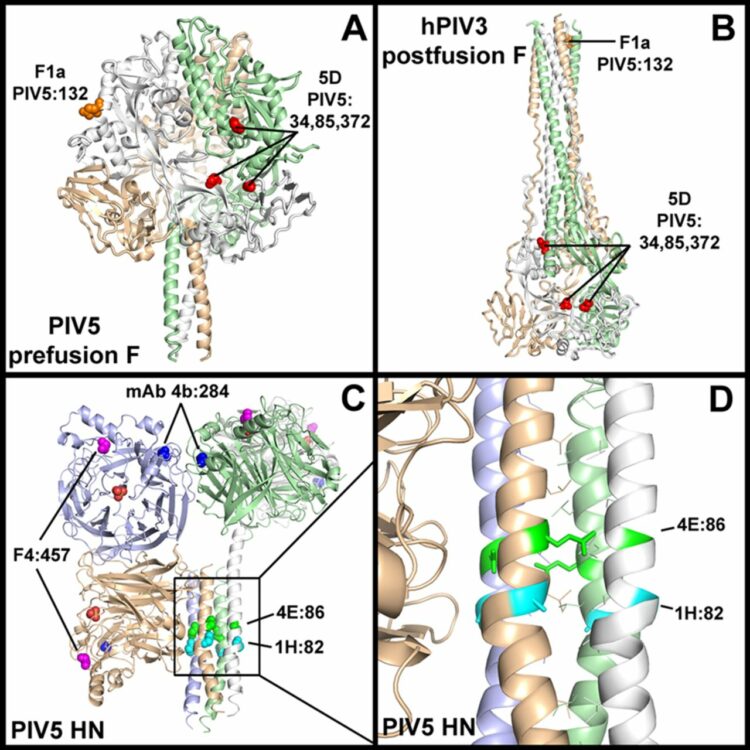
Welch, Brett D; Paduch, Marcin; Leser, George P; Bergman, Zachary; Kors, Christopher A; Paterson, Reay G; Jardetzky, Theodore S; Kossiakoff, Anthony A; Lamb, Robert A
Probing the functions of the paramyxovirus glycoproteins F and HN with a panel of synthetic antibodies Journal Article
In: J Virol, vol. 88, no. 20, pp. 11713–11725, 2014, ISSN: 1098-5514.
@article{pmid25122782,
title = {Probing the functions of the paramyxovirus glycoproteins F and HN with a panel of synthetic antibodies},
author = {Brett D Welch and Marcin Paduch and George P Leser and Zachary Bergman and Christopher A Kors and Reay G Paterson and Theodore S Jardetzky and Anthony A Kossiakoff and Robert A Lamb},
doi = {10.1128/JVI.01707-14},
issn = {1098-5514},
year = {2014},
date = {2014-10-01},
urldate = {2014-10-01},
journal = {J Virol},
volume = {88},
number = {20},
pages = {11713--11725},
abstract = {Paramyxoviruses are enveloped negative-strand RNA viruses that are significant human and animal pathogens. Most paramyxoviruses infect host cells via the concerted action of a tetrameric attachment protein (variously called HN, H, or G) that binds either sialic acid or protein receptors on target cells and a trimeric fusion protein (F) that merges the viral envelope with the plasma membrane at neutral pH. F initially folds to a metastable prefusion conformation that becomes activated via a cleavage event during cellular trafficking. Upon receptor binding, the attachment protein, which consists of a globular head anchored to the membrane via a helical tetrameric stalk, triggers a major conformation change in F which results in fusion of virus and host cell membranes. We recently proposed a model for F activation in which the attachment protein head domains move following receptor binding to expose HN stalk residues critical for triggering F. To test the model in the context of wild-type viral glycoproteins, we used a restricted-diversity combinatorial Fab library and phage display to rapidly generate synthetic antibodies (sAbs) against multiple domains of the paramyxovirus parainfluenza 5 (PIV5) pre- and postfusion F and HN. As predicted by the model, sAbs that bind to the critical F-triggering region of the HN stalk do not disrupt receptor binding or neuraminidase (NA) activity but are potent inhibitors of fusion. An inhibitory prefusion F-specific sAb recognized a quaternary antigenic site and may inhibit fusion by preventing F refolding or by blocking the F-HN interaction. Importance: The paramyxovirus family of negative-strand RNA viruses cause significant disease in humans and animals. The viruses bind to cells via their receptor binding protein and then enter cells by fusion of their envelope with the host cell plasma membrane, a process mediated by a metastable viral fusion (F) protein. To understand the steps in viral membrane fusion, a library of synthetic antibodies to F protein and the receptor binding protein was generated in bacteriophage. These antibodies bound to different regions of the F protein and the receptor binding protein, and the location of antibody binding affected different processes in viral entry into cells.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
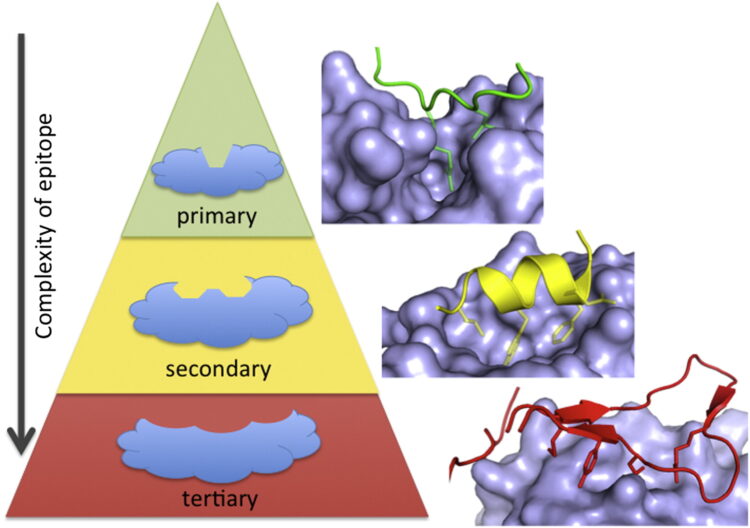
Arkin, Michelle R; Tang, Yinyan; Wells, James A
Small-molecule inhibitors of protein-protein interactions: progressing toward the reality Journal Article
In: Chem Biol, vol. 21, no. 9, pp. 1102–1114, 2014, ISSN: 1879-1301.
@article{pmid25237857,
title = {Small-molecule inhibitors of protein-protein interactions: progressing toward the reality},
author = {Michelle R Arkin and Yinyan Tang and James A Wells},
doi = {10.1016/j.chembiol.2014.09.001},
issn = {1879-1301},
year = {2014},
date = {2014-09-01},
urldate = {2014-09-01},
journal = {Chem Biol},
volume = {21},
number = {9},
pages = {1102--1114},
abstract = {The past 20 years have seen many advances in our understanding of protein-protein interactions (PPIs) and how to target them with small-molecule therapeutics. In 2004, we reviewed some early successes; since then, potent inhibitors have been developed for diverse protein complexes, and compounds are now in clinical trials for six targets. Surprisingly, many of these PPI clinical candidates have efficiency metrics typical of "lead-like" or "drug-like" molecules and are orally available. Successful discovery efforts have integrated multiple disciplines and make use of all the modern tools of target-based discovery-structure, computation, screening, and biomarkers. PPIs become progressively more challenging as the interfaces become more complex, i.e., as binding epitopes are displayed on primary, secondary, or tertiary structures. Here, we review the last 10 years of progress, focusing on the properties of PPI inhibitors that have advanced to clinical trials and prospects for the future of PPI drug discovery.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
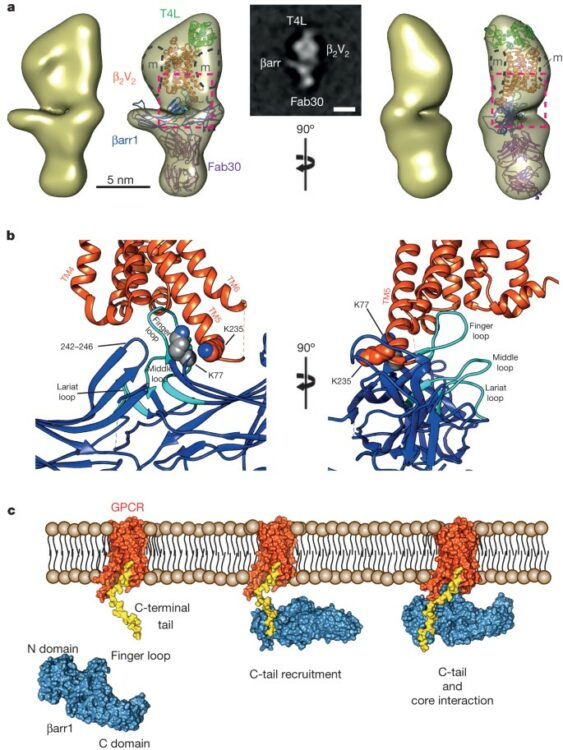
Shukla, Arun K; Westfield, Gerwin H; Xiao, Kunhong; Reis, Rosana I; Huang, Li-Yin; Tripathi-Shukla, Prachi; Qian, Jiang; Li, Sheng; Blanc, Adi; Oleskie, Austin N; Dosey, Anne M; Su, Min; Liang, Cui-Rong; Gu, Ling-Ling; Shan, Jin-Ming; Chen, Xin; Hanna, Rachel; Choi, Minjung; Yao, Xiao Jie; Klink, Bjoern U; Kahsai, Alem W; Sidhu, Sachdev S; Koide, Shohei; Penczek, Pawel A; Kossiakoff, Anthony A; Woods, Virgil L; Kobilka, Brian K; Skiniotis, Georgios; Lefkowitz, Robert J
Visualization of arrestin recruitment by a G-protein-coupled receptor Journal Article
In: Nature, vol. 512, no. 7513, pp. 218–222, 2014, ISSN: 1476-4687.
@article{pmid25043026,
title = {Visualization of arrestin recruitment by a G-protein-coupled receptor},
author = {Arun K Shukla and Gerwin H Westfield and Kunhong Xiao and Rosana I Reis and Li-Yin Huang and Prachi Tripathi-Shukla and Jiang Qian and Sheng Li and Adi Blanc and Austin N Oleskie and Anne M Dosey and Min Su and Cui-Rong Liang and Ling-Ling Gu and Jin-Ming Shan and Xin Chen and Rachel Hanna and Minjung Choi and Xiao Jie Yao and Bjoern U Klink and Alem W Kahsai and Sachdev S Sidhu and Shohei Koide and Pawel A Penczek and Anthony A Kossiakoff and Virgil L Woods and Brian K Kobilka and Georgios Skiniotis and Robert J Lefkowitz},
doi = {10.1038/nature13430},
issn = {1476-4687},
year = {2014},
date = {2014-08-01},
urldate = {2014-08-01},
journal = {Nature},
volume = {512},
number = {7513},
pages = {218--222},
abstract = {G-protein-coupled receptors (GPCRs) are critically regulated by β-arrestins, which not only desensitize G-protein signalling but also initiate a G-protein-independent wave of signalling. A recent surge of structural data on a number of GPCRs, including the β2 adrenergic receptor (β2AR)-G-protein complex, has provided novel insights into the structural basis of receptor activation. However, complementary information has been lacking on the recruitment of β-arrestins to activated GPCRs, primarily owing to challenges in obtaining stable receptor-β-arrestin complexes for structural studies. Here we devised a strategy for forming and purifying a functional human β2AR-β-arrestin-1 complex that allowed us to visualize its architecture by single-particle negative-stain electron microscopy and to characterize the interactions between β2AR and β-arrestin 1 using hydrogen-deuterium exchange mass spectrometry (HDX-MS) and chemical crosslinking. Electron microscopy two-dimensional averages and three-dimensional reconstructions reveal bimodal binding of β-arrestin 1 to the β2AR, involving two separate sets of interactions, one with the phosphorylated carboxy terminus of the receptor and the other with its seven-transmembrane core. Areas of reduced HDX together with identification of crosslinked residues suggest engagement of the finger loop of β-arrestin 1 with the seven-transmembrane core of the receptor. In contrast, focal areas of raised HDX levels indicate regions of increased dynamics in both the N and C domains of β-arrestin 1 when coupled to the β2AR. A molecular model of the β2AR-β-arrestin signalling complex was made by docking activated β-arrestin 1 and β2AR crystal structures into the electron microscopy map densities with constraints provided by HDX-MS and crosslinking, allowing us to obtain valuable insights into the overall architecture of a receptor-arrestin complex. The dynamic and structural information presented here provides a framework for better understanding the basis of GPCR regulation by arrestins.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
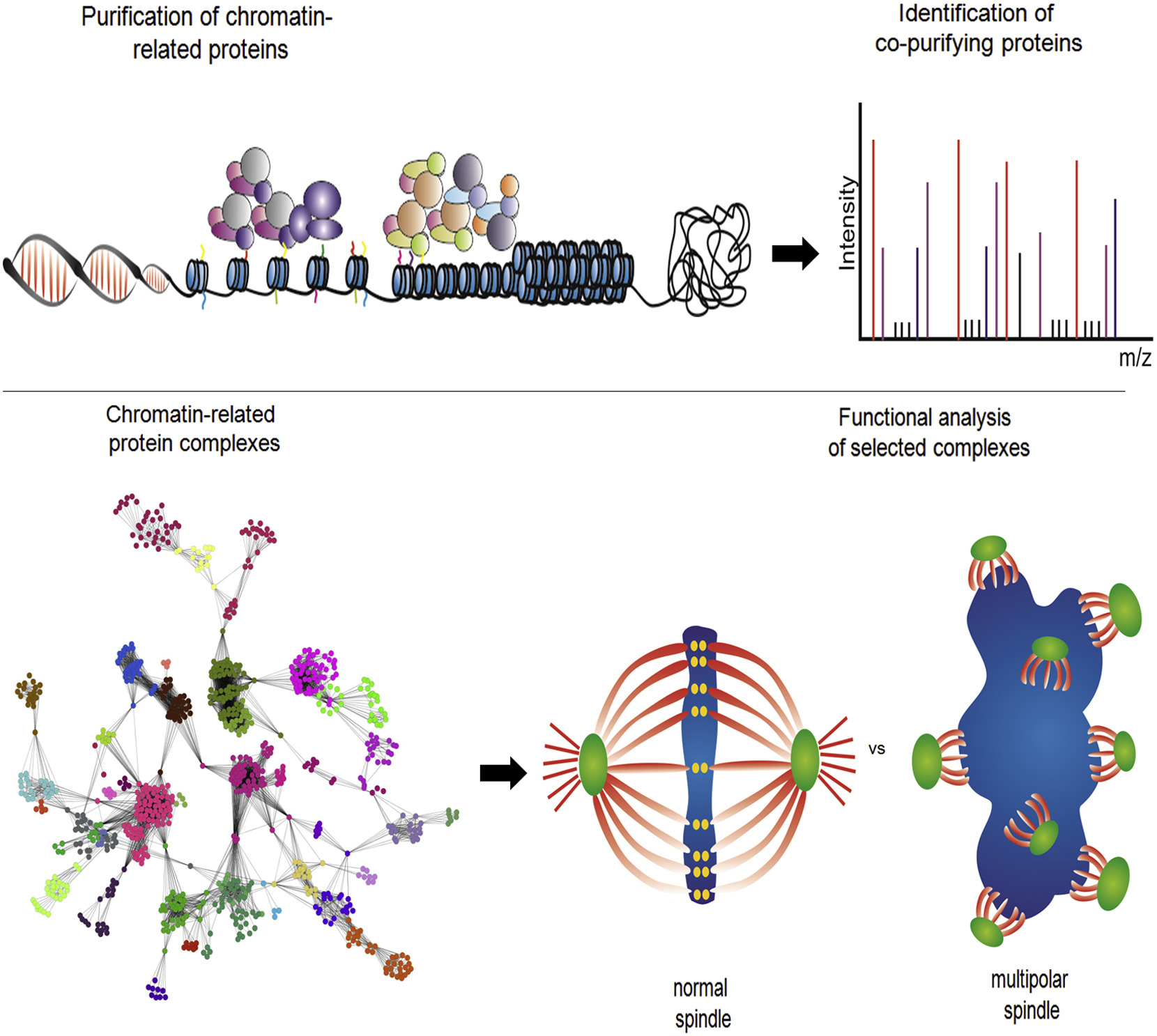
Marcon, Edyta; Ni, Zuyao; Pu, Shuye; Turinsky, Andrei L; Trimble, Sandra Smiley; Olsen, Jonathan B; Silverman-Gavrila, Rosalind; Silverman-Gavrila, Lorelei; Phanse, Sadhna; Guo, Hongbo; Zhong, Guoqing; Guo, Xinghua; Young, Peter; Bailey, Swneke; Roudeva, Denitza; Zhao, Dorothy; Hewel, Johannes; Li, Joyce; Gräslund, Susanne; Paduch, Marcin; Kossiakoff, Anthony A; Lupien, Mathieu; Emili, Andrew; Wodak, Shoshana J; Greenblatt, Jack
Human-chromatin-related protein interactions identify a demethylase complex required for chromosome segregation Journal Article
In: Cell Rep, vol. 8, no. 1, pp. 297–310, 2014, ISSN: 2211-1247.
@article{pmid24981860,
title = {Human-chromatin-related protein interactions identify a demethylase complex required for chromosome segregation},
author = {Edyta Marcon and Zuyao Ni and Shuye Pu and Andrei L Turinsky and Sandra Smiley Trimble and Jonathan B Olsen and Rosalind Silverman-Gavrila and Lorelei Silverman-Gavrila and Sadhna Phanse and Hongbo Guo and Guoqing Zhong and Xinghua Guo and Peter Young and Swneke Bailey and Denitza Roudeva and Dorothy Zhao and Johannes Hewel and Joyce Li and Susanne Gräslund and Marcin Paduch and Anthony A Kossiakoff and Mathieu Lupien and Andrew Emili and Shoshana J Wodak and Jack Greenblatt},
doi = {10.1016/j.celrep.2014.05.050},
issn = {2211-1247},
year = {2014},
date = {2014-07-01},
urldate = {2014-07-01},
journal = {Cell Rep},
volume = {8},
number = {1},
pages = {297--310},
abstract = {Chromatin regulation is driven by multicomponent protein complexes, which form functional modules. Deciphering the components of these modules and their interactions is central to understanding the molecular pathways these proteins are regulating, their functions, and their relation to both normal development and disease. We describe the use of affinity purifications of tagged human proteins coupled with mass spectrometry to generate a protein-protein interaction map encompassing known and predicted chromatin-related proteins. On the basis of 1,394 successful purifications of 293 proteins, we report a high-confidence (85% precision) network involving 11,464 protein-protein interactions among 1,738 different human proteins, grouped into 164 often overlapping protein complexes with a particular focus on the family of JmjC-containing lysine demethylases, their partners, and their roles in chromatin remodeling. We show that RCCD1 is a partner of histone H3K36 demethylase KDM8 and demonstrate that both are important for cell-cycle-regulated transcriptional repression in centromeric regions and accurate mitotic division.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
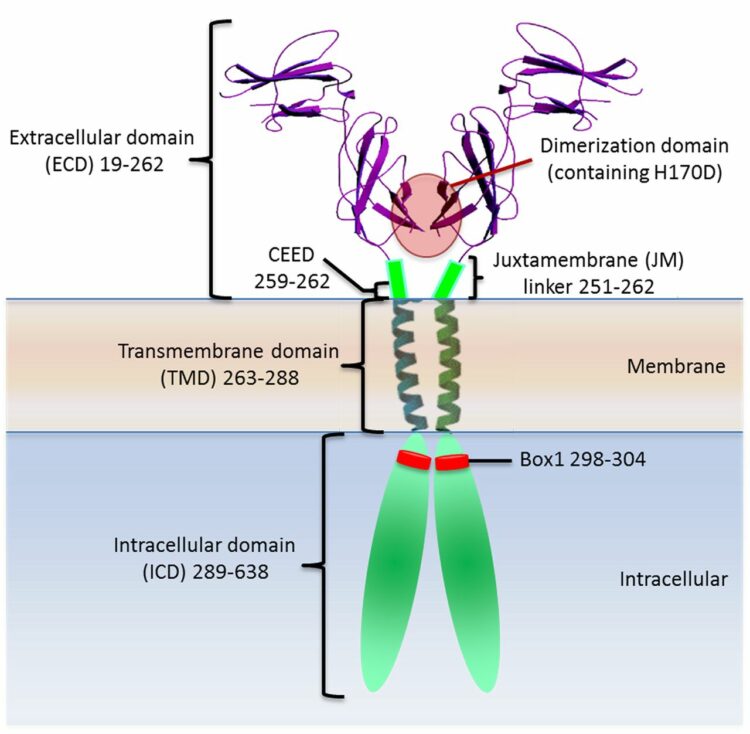
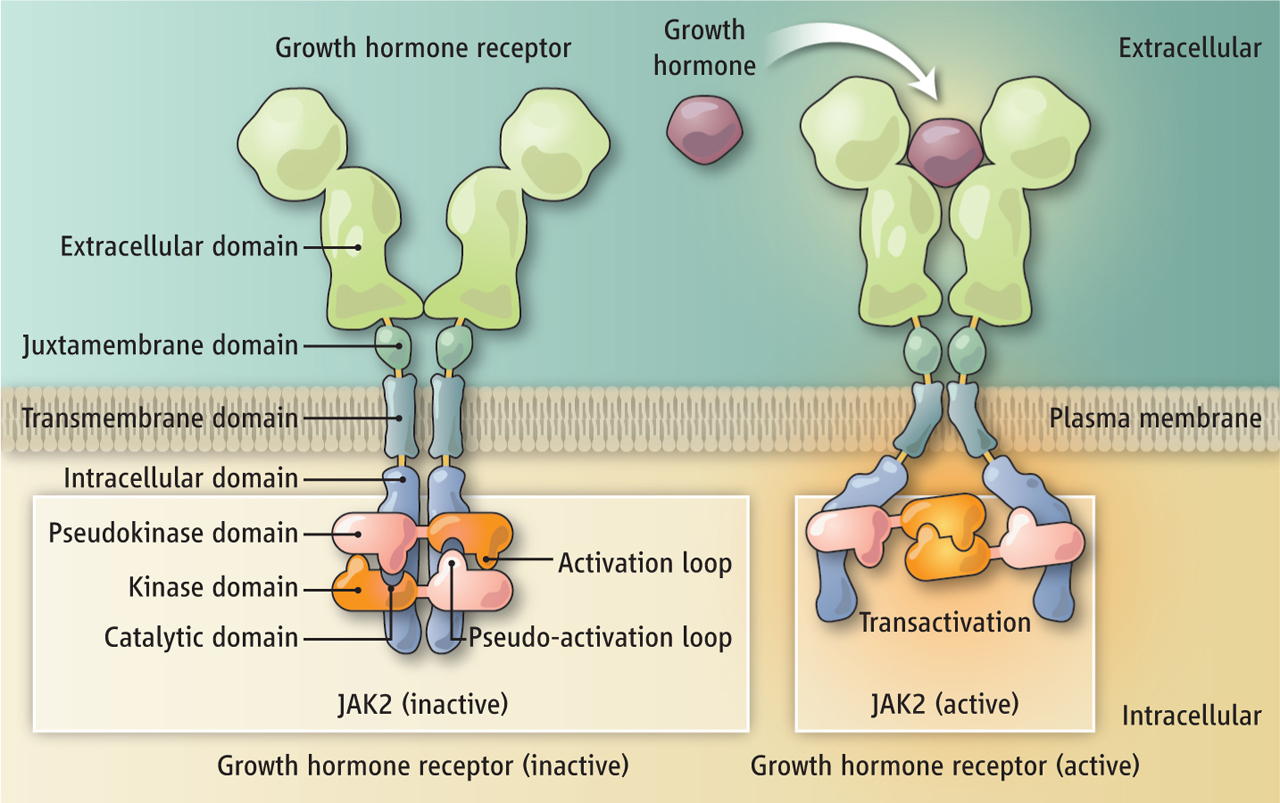
Brooks, Andrew J; Dai, Wei; O'Mara, Megan L; Abankwa, Daniel; Chhabra, Yash; Pelekanos, Rebecca A; Gardon, Olivier; Tunny, Kathryn A; Blucher, Kristopher M; Morton, Craig J; Parker, Michael W; Sierecki, Emma; Gambin, Yann; Gomez, Guillermo A; Alexandrov, Kirill; Wilson, Ian A; Doxastakis, Manolis; Mark, Alan E; Waters, Michael J
Mechanism of activation of protein kinase JAK2 by the growth hormone receptor Journal Article
In: Science, vol. 344, no. 6185, pp. 1249783, 2014, ISSN: 1095-9203.
@article{pmid24833397,
title = {Mechanism of activation of protein kinase JAK2 by the growth hormone receptor},
author = {Andrew J Brooks and Wei Dai and Megan L O'Mara and Daniel Abankwa and Yash Chhabra and Rebecca A Pelekanos and Olivier Gardon and Kathryn A Tunny and Kristopher M Blucher and Craig J Morton and Michael W Parker and Emma Sierecki and Yann Gambin and Guillermo A Gomez and Kirill Alexandrov and Ian A Wilson and Manolis Doxastakis and Alan E Mark and Michael J Waters},
doi = {10.1126/science.1249783},
issn = {1095-9203},
year = {2014},
date = {2014-05-01},
urldate = {2014-05-01},
journal = {Science},
volume = {344},
number = {6185},
pages = {1249783},
abstract = {Signaling from JAK (Janus kinase) protein kinases to STAT (signal transducers and activators of transcription) transcription factors is key to many aspects of biology and medicine, yet the mechanism by which cytokine receptors initiate signaling is enigmatic. We present a complete mechanistic model for activation of receptor-bound JAK2, based on an archetypal cytokine receptor, the growth hormone receptor. For this, we used fluorescence resonance energy transfer to monitor positioning of the JAK2 binding motif in the receptor dimer, substitution of the receptor extracellular domains with Jun zippers to control the position of its transmembrane (TM) helices, atomistic modeling of TM helix movements, and docking of the crystal structures of the JAK2 kinase and its inhibitory pseudokinase domain with an opposing kinase-pseudokinase domain pair. Activation of the receptor dimer induced a separation of its JAK2 binding motifs, driven by a ligand-induced transition from a parallel TM helix pair to a left-handed crossover arrangement. This separation leads to removal of the pseudokinase domain from the kinase domain of the partner JAK2 and pairing of the two kinase domains, facilitating trans-activation. This model may well generalize to other class I cytokine receptors.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Wells, James A; Kossiakoff, Anthony A
Cell biology. New tricks for an old dimer Journal Article
In: Science, vol. 344, no. 6185, pp. 703–704, 2014, ISSN: 1095-9203.
@article{pmid24833381,
title = {Cell biology. New tricks for an old dimer},
author = {James A Wells and Anthony A Kossiakoff},
doi = {10.1126/science.1254799},
issn = {1095-9203},
year = {2014},
date = {2014-05-01},
urldate = {2014-05-01},
journal = {Science},
volume = {344},
number = {6185},
pages = {703--704},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
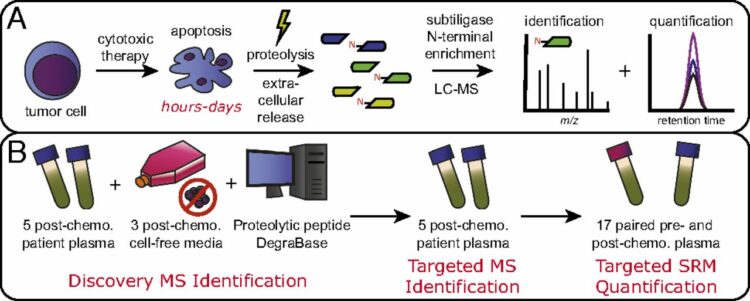
Wiita, Arun P; Hsu, Gerald W; Lu, Chuanyi M; Esensten, Jonathan H; Wells, James A
Circulating proteolytic signatures of chemotherapy-induced cell death in humans discovered by N-terminal labeling Journal Article
In: Proc Natl Acad Sci U S A, vol. 111, no. 21, pp. 7594–7599, 2014, ISSN: 1091-6490.
@article{pmid24821784,
title = {Circulating proteolytic signatures of chemotherapy-induced cell death in humans discovered by N-terminal labeling},
author = {Arun P Wiita and Gerald W Hsu and Chuanyi M Lu and Jonathan H Esensten and James A Wells},
doi = {10.1073/pnas.1405987111},
issn = {1091-6490},
year = {2014},
date = {2014-05-01},
urldate = {2014-05-01},
journal = {Proc Natl Acad Sci U S A},
volume = {111},
number = {21},
pages = {7594--7599},
abstract = {It is known that many chemotherapeutics induce cellular apoptosis over hours to days. During apoptosis, numerous cellular proteases are activated, most canonically the caspases. We speculated that detection of proteolytic fragments released from apoptotic cells into the peripheral blood may serve as a unique indicator of chemotherapy-induced cell death. Here we used an enzymatic labeling process to positively enrich free peptide α-amines in the plasma of hematologic malignancy patients soon after beginning treatment. This N-terminomic approach largely avoids interference by high-abundance proteins that complicate traditional plasma proteomic analyses. Significantly, by mass spectrometry methods, we found strong biological signatures of apoptosis directly in the postchemotherapy plasma, including numerous caspase-cleaved peptides as well as relevant peptides from apoptotic and cell-stress proteins second mitochondria-derived activator of caspases, HtrA serine peptidase 2, and activating transcription factor 6. We also treated hematologic cancer cell lines with clinically relevant chemotherapeutics and monitored proteolytic fragments released into the media. Remarkably, many of these peptides coincided with those found in patient samples. Overall, we identified 153 proteolytic peptides in postchemotherapy patient plasma as potential indicators of cellular apoptosis. Through targeted quantitative proteomics, we verified that many of these peptides were indeed increased post- vs. prechemotherapy in additional patients. Our findings reveal that numerous proteolytic fragments are released from dying tumor cells. Monitoring posttreatment proteolysis may lead to a novel class of inexpensive, rapid biomarkers of cell death.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
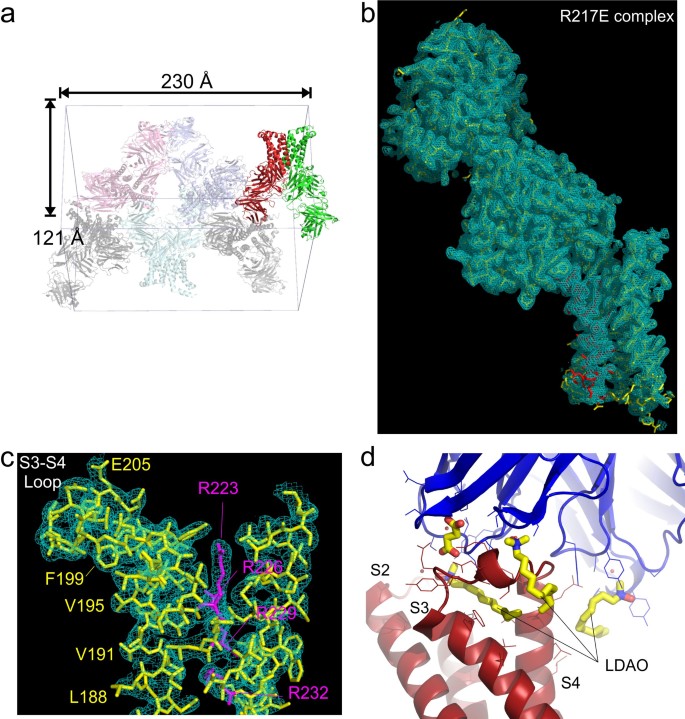
Li, Qufei; Wanderling, Sherry; Paduch, Marcin; Medovoy, David; Singharoy, Abhishek; McGreevy, Ryan; Villalba-Galea, Carlos A; Hulse, Raymond E; Roux, Benoît; Schulten, Klaus; Kossiakoff, Anthony; Perozo, Eduardo
Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain Journal Article
In: Nat Struct Mol Biol, vol. 21, no. 3, pp. 244–252, 2014, ISSN: 1545-9985.
@article{pmid24487958,
title = {Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain},
author = {Qufei Li and Sherry Wanderling and Marcin Paduch and David Medovoy and Abhishek Singharoy and Ryan McGreevy and Carlos A Villalba-Galea and Raymond E Hulse and Benoît Roux and Klaus Schulten and Anthony Kossiakoff and Eduardo Perozo},
doi = {10.1038/nsmb.2768},
issn = {1545-9985},
year = {2014},
date = {2014-03-01},
urldate = {2014-03-01},
journal = {Nat Struct Mol Biol},
volume = {21},
number = {3},
pages = {244--252},
abstract = {The transduction of transmembrane electric fields into protein motion has an essential role in the generation and propagation of cellular signals. Voltage-sensing domains (VSDs) carry out these functions through reorientations of positive charges in the S4 helix. Here, we determined crystal structures of the Ciona intestinalis VSD (Ci-VSD) in putatively active and resting conformations. S4 undergoes an ~5-Å displacement along its main axis, accompanied by an ~60° rotation. This movement is stabilized by an exchange in countercharge partners in helices S1 and S3 that generates an estimated net charge transfer of ~1 eo. Gating charges move relative to a ''hydrophobic gasket' that electrically divides intra- and extracellular compartments. EPR spectroscopy confirms the limited nature of S4 movement in a membrane environment. These results provide an explicit mechanism for voltage sensing and set the basis for electromechanical coupling in voltage-dependent enzymes and ion channels.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
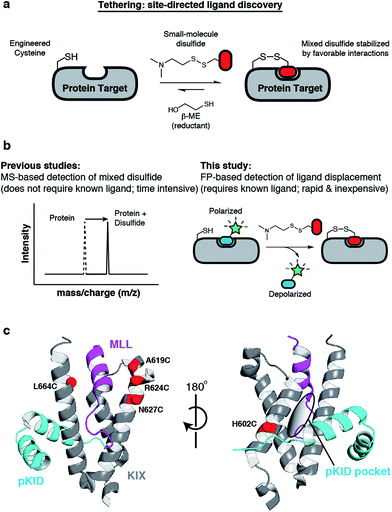
Lodge, Jean M; Rettenmaier, T Justin; Wells, James A; Pomerantz, William C; Mapp, Anna K
FP Tethering: a screening technique to rapidly identify compounds that disrupt protein-protein interactions Journal Article
In: Medchemcomm, vol. 5, pp. 370–375, 2014, ISSN: 2040-2503.
@article{pmid24795804,
title = {FP Tethering: a screening technique to rapidly identify compounds that disrupt protein-protein interactions},
author = {Jean M Lodge and T Justin Rettenmaier and James A Wells and William C Pomerantz and Anna K Mapp},
doi = {10.1039/C3MD00356F},
issn = {2040-2503},
year = {2014},
date = {2014-03-01},
urldate = {2014-03-01},
journal = {Medchemcomm},
volume = {5},
pages = {370--375},
abstract = {Tethering is a screening technique for discovering small-molecule fragments that bind to pre-determined sites via formation of a disulphide bond. Tethering screens traditionally rely upon mass spectrometry to detect disulphide bind formation, which requires a time-consuming liquid chromatography step. Here we show that Tethering can be performed rapidly and inexpensively using a homogenous fluorescence polarization (FP) assay that detects displacement of a peptide ligand from the protein target as an indirect readout of disulphide formation. We apply this method, termed FP Tethering, to identify fragments that disrupt the protein-protein interaction between the KIX domain of the transcriptional coactivator CBP and the transcriptional activator peptide pKID.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
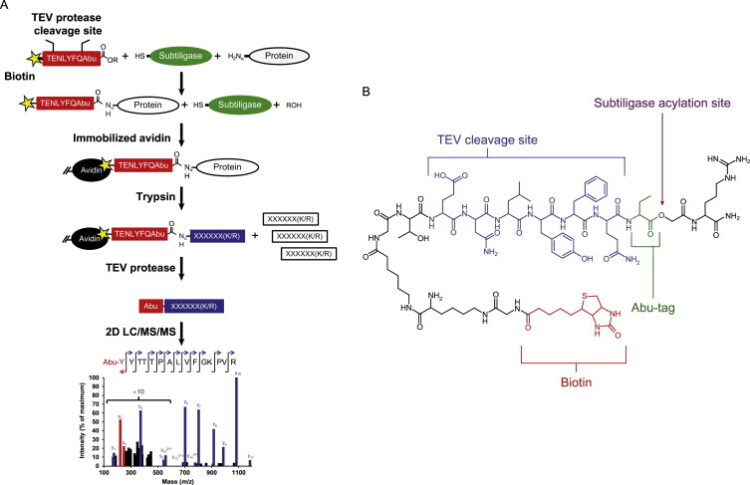
Wiita, Arun P; Seaman, Julia E; Wells, James A
Global analysis of cellular proteolysis by selective enzymatic labeling of protein N-termini Journal Article
In: Methods Enzymol, vol. 544, pp. 327–358, 2014, ISSN: 1557-7988.
@article{pmid24974296,
title = {Global analysis of cellular proteolysis by selective enzymatic labeling of protein N-termini},
author = {Arun P Wiita and Julia E Seaman and James A Wells},
doi = {10.1016/B978-0-12-417158-9.00013-3},
issn = {1557-7988},
year = {2014},
date = {2014-01-01},
urldate = {2014-01-01},
journal = {Methods Enzymol},
volume = {544},
pages = {327--358},
abstract = {Proteolysis is a critical modification leading to alteration of protein function with important outcomes in many biological processes. However, for the majority of proteases, we have an incomplete understanding of both cellular substrates and downstream effects. Here, we describe detailed protocols and applications for using the rationally engineered peptide ligase, subtiligase, to specifically label and capture protein N-termini generated by proteases either induced or added to complex biological samples. This method allows identification of the protein targets as well as their precise cleavage locations. This approach has revealed >8000 proteolytic sites in healthy and apoptotic cells including >1700 caspase cleavages. One can further determine substrate preferences through rate analysis with quantitative mass spectrometry, physiological substrate specificities, and even infer the identity of proteases operating in the cell. In this chapter, we also describe how this experimental method can be generalized to investigate proteolysis in any biological sample.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
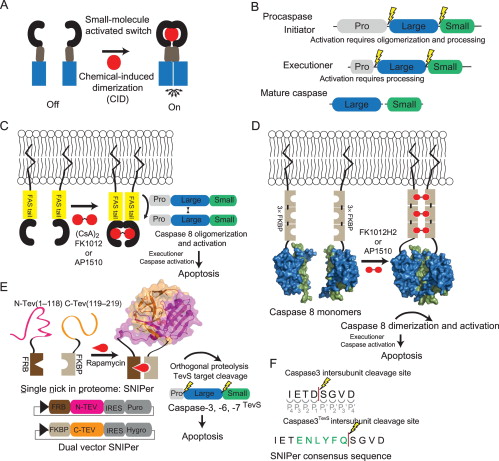
Morgan, Charles W; Julien, Olivier; Unger, Elizabeth K; Shah, Nirao M; Wells, James A
Turning on caspases with genetics and small molecules Journal Article
In: Methods Enzymol, vol. 544, pp. 179–213, 2014, ISSN: 1557-7988.
@article{pmid24974291,
title = {Turning on caspases with genetics and small molecules},
author = {Charles W Morgan and Olivier Julien and Elizabeth K Unger and Nirao M Shah and James A Wells},
doi = {10.1016/B978-0-12-417158-9.00008-X},
issn = {1557-7988},
year = {2014},
date = {2014-01-01},
urldate = {2014-01-01},
journal = {Methods Enzymol},
volume = {544},
pages = {179--213},
abstract = {Caspases, aspartate-specific cysteine proteases, have fate-determining roles in many cellular processes including apoptosis, differentiation, neuronal remodeling, and inflammation (for review, see Yuan & Kroemer, 2010). There are a dozen caspases in humans alone, yet their individual contributions toward these phenotypes are not well understood. Thus, there has been considerable interest in activating individual caspases or using their activity to drive these processes in cells and animals. We envision that such experimental control of caspase activity can not only afford novel insights into fundamental biological problems but may also enable new models for disease and suggest possible routes to therapeutic intervention. In particular, localized, genetic, and small-molecule-controlled caspase activation has the potential to target the desired cell type in a tissue. Suppression of caspase activation is one of the hallmarks of cancer and thus there has been significant enthusiasm for generating selective small-molecule activators that could bypass upstream mutational events that prevent apoptosis. Here, we provide a practical guide that investigators have devised, using genetics or small molecules, to activate specific caspases in cells or animals. Additionally, we show genetically controlled activation of an executioner caspase to target the function of a defined group of neurons in the adult mammalian brain.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}