Welcome to the Recombinant Antibody Network
The Recombinant Antibody Network is a consortium of highly integrated technology centers at UCSF, the University of Chicago, and the University of Toronto, unified under a common goal to generate therapeutic grade recombinant antibodies at a proteome wide scale for biology and biomedicine.
Given that over half the human proteome is not annotated and that functional antibodies are not reliably available, a complete set of validated antibodies would greatly advance all areas of biology, including cancer therapy and infectious disease control. To undertake these challenges, RAN is systematically and comprehensively profiling families of protein targets using novel, modern high-throughput in vitro technology.
Latest Publications
O'Leary K M; Slezak T; Kossiakoff A A
Conformation-specific synthetic intrabodies modulate mTOR signaling with subcellular spatial resolution Journal Article
In: Proc Natl Acad Sci U S A, vol. 122, no. 24, pp. e2424679122, 2025, ISSN: 1091-6490.
@article{pmid40489625,
title = {Conformation-specific synthetic intrabodies modulate mTOR signaling with subcellular spatial resolution},
author = {Kelly M O'Leary and Tomasz Slezak and Anthony A Kossiakoff},
doi = {10.1073/pnas.2424679122},
issn = {1091-6490},
year = {2025},
date = {2025-06-01},
urldate = {2025-06-01},
journal = {Proc Natl Acad Sci U S A},
volume = {122},
number = {24},
pages = {e2424679122},
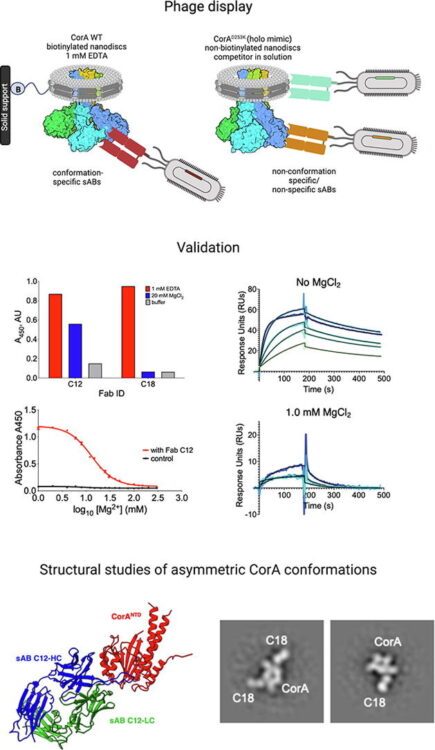
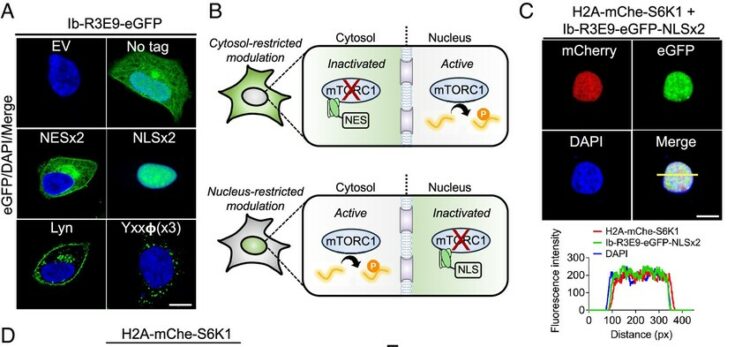
abstract = {Subcellular compartmentalization is integral to the spatial regulation of mechanistic target of rapamycin (mTOR) signaling. However, the biological outputs associated with location-specific mTOR signaling events are poorly understood and challenging to decouple. Here, we engineered synthetic intracellular antibodies (intrabodies) that are capable of modulating mTOR signaling with genetically programmable spatial resolution. Epitope-directed phage display was exploited to generate high affinity synthetic antibody fragments (Fabs) against the FKBP12-Rapamycin binding site of mTOR (mTOR). We determined high-resolution crystal structures of two unique Fabs that discriminate distinct conformational states of mTOR through recognition of its substrate recruitment interface. By leveraging these conformation-specific binders as intracellular probes, we uncovered the structural basis for an allosteric mechanism governing mTOR complex 1 (mTORC1) stability mediated by subtle structural adjustments within mTOR. Furthermore, our results demonstrated that synthetic binders emulate natural substrates by employing divergent yet complementary hydrophobic residues at defined positions, underscoring the broad molecular recognition capability of mTOR. Intracellular signaling studies showed differential time-dependent inhibition of S6 kinase 1 and Akt phosphorylation by genetically encoded intrabodies, thus supporting a mechanism of inhibition analogous to the natural product rapamycin. Finally, we implemented a feasible approach to selectively modulate mTOR signaling in the nucleus through spatially programmed intrabody expression. These findings establish intrabodies as versatile tools for dissecting the conformational regulation of mTORC1 and should be useful to explore how location-specific mTOR signaling influences disease progression.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Adams J J; Bruce H A; Subramania S; Ploder L; Garcia J; Pot I; Blazer L L; Singer A U; Sidhu S S
Synthetic antibodies targeting EphA2 induce diverse signaling-competent clusters with differential activation Journal Article
In: Protein Sci, vol. 34, no. 6, pp. e70145, 2025, ISSN: 1469-896X.
@article{pmid40411427,
title = {Synthetic antibodies targeting EphA2 induce diverse signaling-competent clusters with differential activation},
author = {Jarrett J Adams and Heather A Bruce and Suryasree Subramania and Lynda Ploder and Julia Garcia and Isabelle Pot and Levi L Blazer and Alexander U Singer and Sachdev S Sidhu},
doi = {10.1002/pro.70145},
issn = {1469-896X},
year = {2025},
date = {2025-06-01},
urldate = {2025-06-01},
journal = {Protein Sci},
volume = {34},
number = {6},
pages = {e70145},
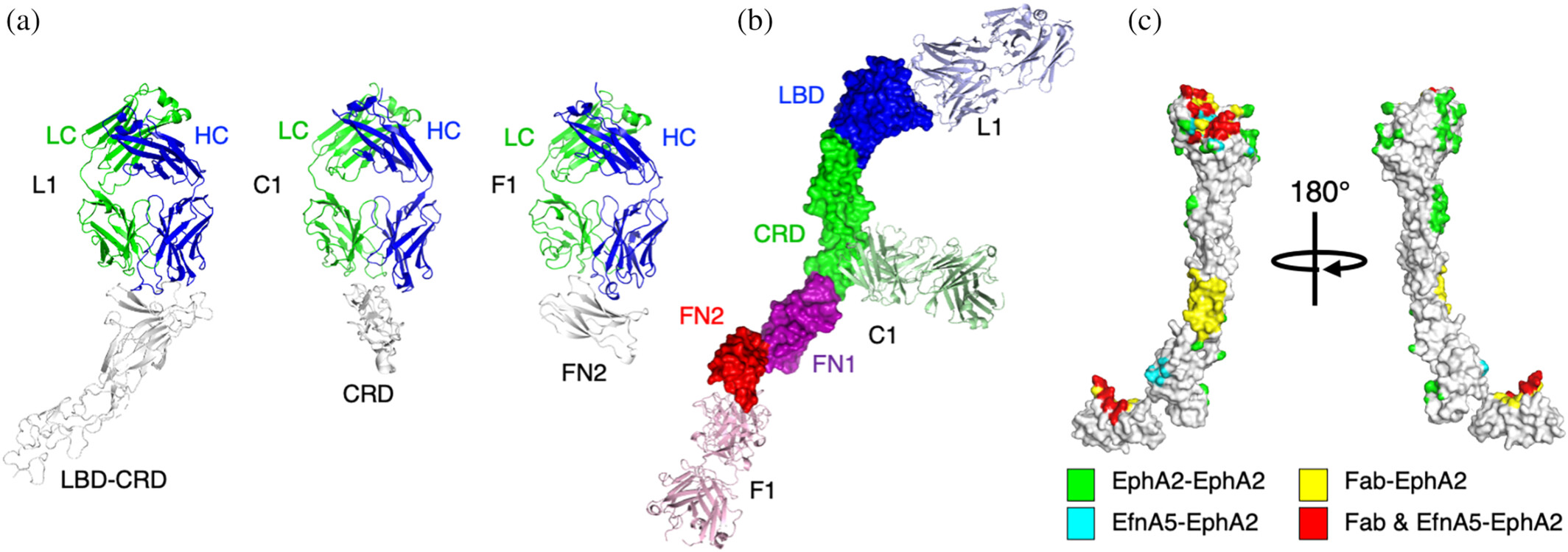
abstract = {The receptor tyrosine kinase EphA2 interacts with ephrin (Efn) ligands to mediate bi-directional signals that drive cellular sorting processes during tissue development. In the context of various cancers, EphA2 can also drive invasive metastatic disease and represents an important target for cancer therapeutics. Natural Efn ligands sterically seed intertwined EphA2 clusters capable of recruiting intracellular kinases to mediate trans-phosphorylation. Synthetic proteins, such as antibodies (Abs), can mimic Efn ligands to trigger EphA2 signaling, leading to receptor internalization and degradation, and enabling intracellular delivery of conjugated drugs. Furthermore, Abs are capable of recruiting EphA2 into clusters distinct from those seeded by Efn. We developed three synthetic Abs targeting distinct EphA2 domains and determined the paratope valency necessary for agonist or antagonist properties of each of the three epitopes. Structural modeling of monovalent Fabs in complex with EphA2 elucidated competitive and non-competitive mechanisms of inhibition of EphA2 canonical signaling. Likewise, modeling of clusters induced by bivalent IgGs elucidated multiple signaling-competent EphA2 clusters capable of triggering a continuum of signaling strengths and provided insights into the requirement for multimerization of EphA2 to trigger phosphorylation. Our study shows how different agonist clusters lead to distinct kinase recruitment efficiencies to modify phosphotyrosine signal strength, and provides a panel of anti-EphA2 Abs as reagents for the development of therapeutics.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Miersch S; Singer A U; Chen C; Fellouse F; Gopalsamy A; Costa L S E; Lacasta A; Chege H; Chege N; Nene V; Sidhu S S
Molecular characterization of a synthetic neutralizing antibody targeting p67 of Theileria parva Journal Article
In: Protein Sci, vol. 34, no. 6, pp. e70153, 2025, ISSN: 1469-896X.
@article{pmid40384600,
title = {Molecular characterization of a synthetic neutralizing antibody targeting p67 of Theileria parva},
author = {Shane Miersch and Alex U Singer and Chao Chen and Fred Fellouse and Anupriya Gopalsamy and Luciano Silva E Costa and Anna Lacasta and Hannah Chege and Naomi Chege and Vishvanath Nene and Sachdev S Sidhu},
doi = {10.1002/pro.70153},
issn = {1469-896X},
year = {2025},
date = {2025-06-01},
urldate = {2025-06-01},
journal = {Protein Sci},
volume = {34},
number = {6},
pages = {e70153},
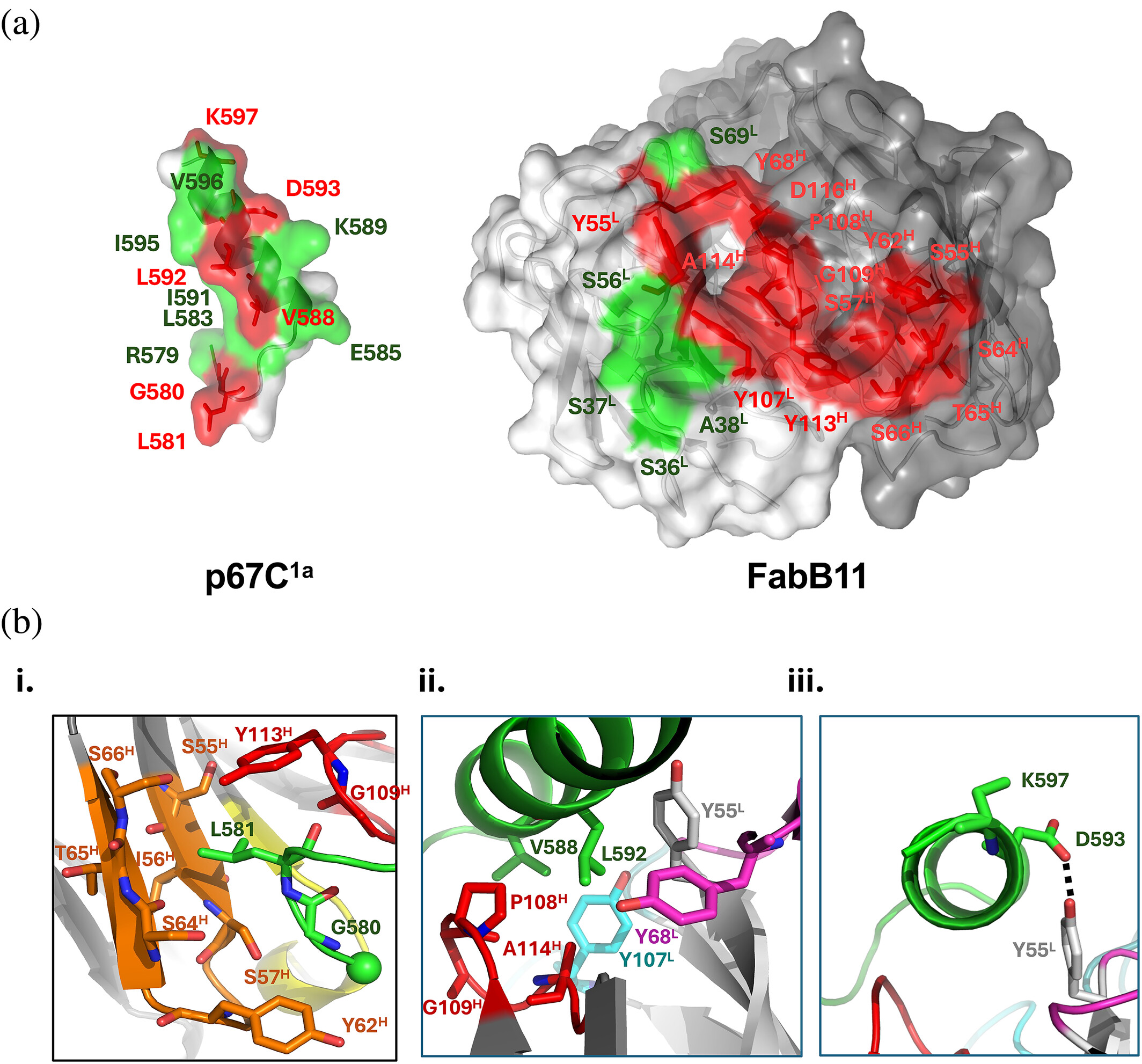
abstract = {The Theileria parva sporozoite surface antigen p67 is a target of the bovine humoral immune response that generates antibodies capable of providing protection against subsequent infection. As a result, p67 has been the subject of efforts aimed at the development of an anti-sporozoite subunit vaccine. Previous studies have identified neutralizing epitopes in the N- and C-terminal regions of the full-length protein and shown that immunization with a C-terminal fragment of p67 (p67C) alone is capable of eliciting protection. To identify additional neutralizing epitopes in p67C, selections were conducted against it using a phage-displayed synthetic antibody library. An antibody that neutralized the sporozoite in vitro was identified, and the crystal structure of a Fab:peptide complex was elucidated. Mutagenesis studies aimed at validating and further characterizing the Fab:peptide interaction identified critical residues involved in binding and neutralization. This study also validates distinct epitopes for previously reported neutralizing antibodies.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Latest News
Recombinant Antibody Network Partners with Bristol Myers Squibb to Develop Novel Therapies
The Recombinant Antibody Network (RAN), a consortium comprising research groups from UC San Francisco, the…
Absolute Antibody Partners with the Recombinant Antibody Network to Facilitate Access to Engineered Recombinant Antibodies
Absolute Antibody Ltd., an industry-leading provider of recombinant antibody products and services, has announced a…
RAN to collaborate with Celgene on cancer therapeutics development
The RAN has recently signed a 3-year $25M agreement with the Celgene Corporation to develop next-generation,…
